Coordination Compounds Class 12 Chemistry Notes
Introduction to Coordination Chemistry or Coordination Compounds
- Coordination compounds are the compounds in which the central metal atom is linked to a number of ions or neutral molecules by coordinate bond.
- Coordinate bond is a covalent type of bond in which both the shared pair of electrons are contributed by one atom.
WERNER’S THEORY OF COORDINATION COMPOUND
Basic postulates of Werner’s theory
- Most of the metallic elements exhibit two types of valence.
- Primary valence or principal valence.
- Secondary valence.
- Every metal tends to satisfy both of its primary and secondary valences.
- Every metal has a fixed number of secondary valences.
- The secondary valence is always directed towards fixed position in space.
Werner’s theory in the light of modern electronic theory of valence.
- According to Werner, two spheres of attraction are present around the metal. The inner zone is coordination sphere, and the outer zone is ionization sphere.
- In terms of modern electronic theory of valence, the coordination sphere is equivalent to the coordination number of the metal ion and the ionization sphere is equivalent to the ionisable or the oxidation state of the metal ion.
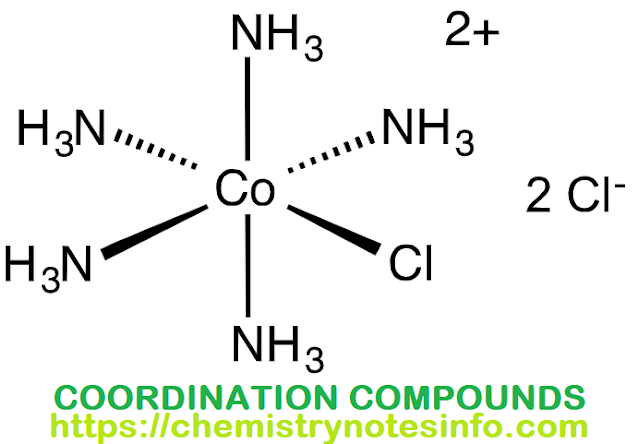
- Werner introduced a square bracket [ ] to enclose the central metal ion and the ligands. This represents coordination sphere. The ions of the ionization sphere are placed outside the square bracket. e.g. [CO(NH3)6]Cl3, in this complex the coordination sphere includes six ammonia molecules while ionization sphere includes three chloride ions.
Application of Werner’s theory to CO (III) amines in Coordination Compounds Class 12
- [CO(NH3)5]Cl2
- [CO(NH3)5Cl]Cl2
- [CO(NH3)4Cl2]Cl
- [CO(NH3)3Cl3]
Experimental observation to CO (III) amines
- The primary valence (or oxidation state) of cobalt is 3 while secondary valence (or coordination number) is 6.
- When cobalt amines are heated with hydrochloric acid at 373 K ammonia is not removed (evolved). In all these compounds ammonia molecules are seen to be firmly bound.
- All these compounds are found to differ in their electrical properties.
- All these compounds are differing in their reactivity towards silver nitrate.
LIGANDS
Types of Ligands
1. Mono or unidentate ligands
2. Poly multidentate ligands
- Bidentate ligands
- Tridentate ligands
- Tetrodentate ligands
- Hexadentate ligands
3. Ambidentate ligands
COORDINATION NUMBER (CN)
Metal ion and coordination number
| Metal ion | Oxidation state | CN | Examples |
| Ag+ | +1 | 2 | [Ag (NH3)2]+ |
| Cu2+, Ni2+, Zn2+, Cd2+, Hg2+, Pt2+ | +2 | 4 | [Cu(NH3)4]2+ |
| Fe3+, CO3+, Cr3+ | +3 | 6 | [CO(NH3)6]3+ |
| Sn4+, Pt4+ | +4 | 6 | [Pt(NH3)6]4+ |
| MO4+ | +4 | 8 | [MO(CN)8]4- |
The coordination number of the metal ion is influenced by –
Homoleptic and heteroptic complexs
Cationic, Anionic and Neutral complexes
A] Cationic complex
B] Anionic complex
C] Neutral complex
Charge number of complex ion
Coordination polyhedron
Double salt and coordination compounds
Characteristics of complex ions
- Generally a transition metal ion is the central metal ion in a complex.
- An ion loses its individual properties and acquires the properties of the complex ion, which it forms.
- The complex ion can dissociate to a slight extent in the solution however it retains its identity.
- The algebraic sum of the charges of constituent ion is the net charge of complex ion.
- The stability of chelate complex is higher than those complexes which are similar but non – chelated.
Effective Atomic Number (EAN)
EAN of few metal ions
| metal | Complex | Z | X | Y | EAN |
| Ni | Ni(CO) | 28 | 0 | 8 | 36 |
| Fe | [Fe(CN)6]4- | 26 | 2 | 12 | 36 |
| CO | [CO(NH3)6]3+ | 27 | 3 | 12 | 36 |
| Zn | [Zn(NH3)4]2+ | 30 | 2 | 8 | 36 |
| Pt | [Pt(NH3)6]4+ | 78 | 4 | 12 | 86 |
ISOMERISM IN COORDINATION COMPOUND
A] Stereoisomerism
Types of Stereoisomerism
I] Geometrical isomerism
- Geometrical isomerism is due to difference in the spatial arrangement of atoms or groups of atoms around the central metal atom or ion.
- This occurs in heteroleptic complexes.
- When two similar groups (ligands) occupy adjacent positions, the isomer is called CiS and when two similar groups are arranged opposite to one another, the isomer is called trans.
- It is present in square planner complexes with coordination number 4 and octahedral complexes with coordination number 6.
- Tetrahedral complexes with coordination number 4 do not show this isomerism as all the four position are equivalent.
II] Optical Isomerism
- Optical isomerism in coordination compounds arises out of chirality of coordination entity.
- Chiral compounds do not have any element of symmetry and are optically active.
- Optical isomerism are non-super imposable mirror images of each other and are called enantiomers.
- Optical isomers rotate the plane of plane polarized light. The isomers which rotates the plane of plane polarized light in the clockwise direction is known as d – form.
- The isomers which rotate the plane of plane polarized light in the anti – clockwise direction is known as l – form.
- Optical isomerism is not so common in tetrahedral and square planer complexes. However octahedral complexes bidentate ligands show optical isomerism. Some examples of such type of octahedral complexes are as follows:
B] Structural Isomerism
I] Ionization isomerism
II] Linkage Isomerism
III] Coordination Isomerism
IV] Hydrate Isomerism
VALENCE BOND THEORY (VBT)
Salient features of VBT
- A central metal ion present in a complex provides a definite number of vacant orbital’s S, P, and d for the formation of coordination bonds with the ligands.
- The number of vacant orbital’s provided by the central ion is the same as its coordination number.
- These vacant orbital’s undergo hybridization to form same number of hybrid orbital’s.
- Each ligands has at least one orbital containing a lone pair of electron.
- The geometrical shape of the complex ion depends upon the hybridization of the metal orbital’s.
- The vacant hybrid orbital of the metal ion overlaps with filled orbital’s of the ligands to form coordinate bond between metal and ligand.
- The coordination bond is stronger if the overlapping between the orbital is greater.
- If central metal atom contains then it exhibits paramagnetic property and if central metal atom contains no unpaired electron then, it exhibits diamagnetic property.
Structure of complex compounds based on valence bond theory
- [Ni(CO)4]
- [Ni(Cl)4]2-
- [Ni(CN4)]2-
- [CO(NH3)6]3+
- [COF6]3-
Limitation of valence bond theory
- It cannot explain the spectral properties (colors) of complex compounds.
- It cannot explain why some complexes are inner complexes for the some are outer complexes for the same metal ion in the same oxidation state.
- It does not provided quantitative interpretations of thermodynamic or kinetic stabilities of coordination compounds.
- There is no distinction between weak field and strong field ligands.
- It cannot predict if a 4 coordination complex will have tetrahedral or square planar geometry.
- It does not explain the order of reactivity of inner orbital inert complexes of d3, d4, d5 and d6 ions.
- It does not explain magnetic moments.
Crystal Field Theory (CFT)
Salient features of crystal field theory (CFT)
- In a complex the central metal atom or ion is surrounded by various atom or group of atoms called ligands.
- The ligands are either negatively charged ion (Fˉ, Clˉ, CNˉ etc.) or neutral molecules possessing lone pair of electrons (H2O, NH3, etc). In the neutral ligands the most electronegative atom points towards central metal ion.
- Ligands and metal ions act as point charges. Electrostatic interaction is present between electrons of metal and ligand.
- When the ligand approach the metal atom, the electron of the central atom and those of ligands repel each other. These repulsive forces destroy the degeneracy of d – orbital’s and split them into two groups called t2g and eg group.
- Electrons first occupy lower energy t2g level. Electron occupancy is in accordance with Hund’s rule.
- Crystal field theory does not account for covalent character as orbital overlap is not considered.
- Crystal field stabilization energy (CFSE) determines the stability of complex nature. Number of ligands and geometry of the complex determines the magnitude of CFSE.
Weak field ligand and strong field ligand
Spectrochemical series
- The arrangement of ligands in order of their increasing field strengths, i.e. increasing crystal field splitting energy (CFSE) values is called spectrochemical series.
- Spectroscopy is used to determine the values of pairing energy (P) and Δᵒ.
- The spectrochemical series is shown below
Limitation of crystal field theory (CFT)
- It explain only about the central metal ion with d orbital’s but does explain about orbital’s like s and p.
- The theory does not explains about ∏ bonding in complexes.
- In the spectrochemical series water is a stronger ligand than OHˉ which is not explain satisfactorily.
- According to this theory metal ligand bond is ionic, but cannot explain partly covalent nature of the metal – ligand bond.
COLOURS IN COORDINATION COMPOUNDS
- The crystal field theory explains the origin of colour of coordination compounds. Colour is due to d-d transition.
- When complex compound absorb light of particular wavelength, an electron is excited from lower t2g level to higher eg level. Due to this the coordination compound has a complementary colour.
- An octahedral complex [Ti(H2O)6]3+ with d1 configuration with one electron in lower t2g level. Complex absorbs light in the blue green region. The electron is excited from lower t2g level to higher eg level.
- The colour observed is purple colour.
- [Cu(H2O)6]+2 absorbs red light and the observed colour is blue. This is due to transition of one unpaired electron of d9 configuration.
Relation between complex entity and wavelength
| Coordination entity | Wavelength of light absorb | Colour of light absorb | Colour of coordination entity |
| [Ti(H2O)6]3+ | 498 | Blue green | Purple |
| [CO(CN)6]3- | 310 | Ultraviolet | Pale yellow |
| [COCl(NH3)5]2+ | 535 | Yellow | Violet |
| [CO(NH3)5(H2O)]3+ | 500 | Blue green | Red/purple |
| [CO(NH3)6]3+ | 475 | Blue | Yellow orange |
| [Cu(H2O)4]2+ | 600 | Red | blue |
BONDING IN METAL CARBONYL
- There is donation of lone pair of electrons of carbon (of Co) into the suitable empty orbital of the metal atom. This is a dative overlap and forms a sigma M←C bond.
- There is ∏ – overlap involving donation of electrons from filled metal d – orbital’s into vacant antibonding ∏* molecular orbital’s of CO. this result into the formation of M→C∏ bond.
STABILITY OF COORDINATION COMPOUNDS
- The stability of coordination compounds depend on the metal ion and ligands. For thermodynamically stable compounds, this interaction is strong.
- The thermodynamic stability is quantitatively expressed in terms of the stability constant which is an equilibrium constant. For the equilibrium between metal ion and ligand.
- The equilibrium constant for stability constant is expressed as
- High value of stability constant indicates greater thermodynamic stability.
Stability constant for formation of few complex
| System | Stability Constant K |
| Ag+ + 2NH3 ↔ [Ag(NH3)2]+ | 1.6 X 107 |
| Ag+ + 2CN ↔ [Ag(CN)2]– | 5.5 X 1018 |
| Cu2+ + 4NH3 ↔ [Cu(NH3)4]2+ | 4.5 X 1011 |
| Cu2+ + 4CN– ↔ [Cu(CN)4]2- | 2.0 X 1027 |
What is Lewis Acid
A Lewis acid in coordination chemistry is a chemical species or molecule that can accept a pair of electrons to form a coordinate covalent bond. So, we can say that Lewis acids are electron pair acceptors.
Common examples of Lewis acids include:
1. Metal cations: Many metal ions can act as Lewis acids by accepting electron pairs. For example, Al3+ and Fe3+ ions can form coordination complexes by accepting pairs of electrons from Lewis bases.
2. Electron-deficient molecules: Compounds like boron trifluoride (BF3) and boron trichloride (BCl3) readily accept electron pairs, making them Lewis acids.
3. Carbocations: Positively charged carbon ions formed during organic chemical reactions can also act as Lewis acids.
4. Protons (H+): In some cases, hydrogen ions (protons) can act as Lewis acids by accepting electrons to form a covalent bond.
What is Lewis Base
A Lewis base in coordination chemistry is a chemical species or molecule that can donate a pair of electrons to form a coordinate covalent bond with a Lewis acid. So, we can say that Lewis bases are electron pair donors.
Common examples of Lewis bases include:
1. Ammonia (NH3): Ammonia is a classic example of a Lewis base. It has a lone pair of electrons on the nitrogen atom, which it can donate to form a coordinate covalent bond with a Lewis acid.
2. Water (H2O): Water molecules also have two lone pairs of electrons on the oxygen atom, making them capable of acting as Lewis bases.
3. Hydroxide ion (OH⁻): The hydroxide ion, which is a common base in aqueous solutions, has a lone pair of electrons on the oxygen atom.
4. Alkoxide ions: Alkoxides, such as ethoxide (CH3CH2O⁻), have lone pairs on the oxygen atom and can act as Lewis bases.
5. Amines: Organic compounds containing nitrogen atoms with lone pairs, like primary, secondary, and tertiary amines, can function as Lewis bases.
6. Phosphines: Compounds containing phosphorus with lone pairs of electrons can also act as Lewis bases.
Electron Pairing in Coordination Chemistry
Electron pairing in coordination chemistry is very important phenomena. Electron pairing is the formation of coordinate covalent bonds between a Lewis base and a Lewis acid to create a coordination complex.
Coordination complexes are molecules or ions in which a central metal atom or ion is bonded to one or more surrounding molecules or ions, known as ligands. The bonding between the metal and the ligands is typically achieved through the sharing of electrons, and this involves the electron pairing.
What is Oxidation Number
An oxidation number is also known as oxidation state. It is used to show the charge of an atom within a compound or molecule. Oxidation number or oxidation state is the number assigned to an atom to indicate the number of electrons it has gained or lost when it forms a chemical bond with other atoms.
APPLICATION OF COORDINATION COMPOUNDS
- Extraction of metal.
- Analytical chemistry.
- Medicine.
- Electroplating.
- Estimation of hardness of water.
- Modifying the redox behaviour of metal ions.
- Biological system.

![[Pt(NH3)2Cl2] in Coordination compounds chemistry [Pt(NH3)2Cl2] in Coordination compounds chemistry](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhoVn_QTvqLH8VyOpbb8kEqB1hm68bvwoOCcc65X9NuXH-FhaArK5dtWgSrOzWerirMIHtMIbL0NqDtNaysRuJAgKf5DtGl02ZgXvPrwTKPTJrmUPH4RgktUjcrIEZrTeRhO3AkBuepuqTh/w640-h336-rw/Geometrical+isomerism+_+coordination+compound+by+Chemistry+Notes+Info.png)
