Zinc Metalloenzymes BSc Chemistry Notes
Zinc Metalloenzymes (Bioinorganic Chemistry) BSc Chemistry Notes
These are chemistry notes of Zinc Metalloenzymes of bioinorganic chemistry for BSc and MSc chemistry students.
Enzymes
Enzymes are large protein molecules that catalyze large number of biochemical reactions. They increase the rate of biochemical reactions about 106 times compared to the uncatalyzed rate.
They lower the activation energy for the formation of one product rather than other and therefore are highly specific.
Metalloenzymes
- A metalloenzyme is an enzymatic protein in which a metal as metal ion is embedded in the cavity of the enzyme and forms strong bonds with the donor atoms of the protein. The donor atoms of proteins may be either soft base as sulphur or hard bases such oxygen and nitrogen. In the similar way the metals may be either soft metal such as Cu+, Hg+ and Cd+ or hard such as Fe3+ and Zn2+.
- The protein part is called as an apoenzyme and a metal ion or complex metal ion is called a prosthetic group.
Zinc Metalloenzyme
- Zinc has a highly concentrated charge in comparison to its relatively small ionic radius (0.65A°) and binds modestly to anions such as carboxylates and phosphates.
- Its second characteristic is its high affinity for electrons, making it a strong Lewis acid, similar to copper and nickel.
- It does not show variable valence, which might lead to it being preferred quite simply because it does not introduce the risk of free radical reactions.
- Zinc is the second most abundant trace element in the human body. An average adult has about 3 g of Zn, corresponding to a concentration of zinc of about 0.6 mM, most of which (some 95%) is intracellular.
- Zinc is essential for growth and development in all forms of life, has been proposed to have beneficial therapeutic and preventative effects on infectious diseases, including a shortening of the length of the common cold in man.
- Zinc is found in more than 300 enzymes, where it plays both a catalytic and a structural role. It is the only metal to have representatives in each of the six fundamental classes of enzymes recognised by the International Union of Biochemistry:
- Oxidoreductases like alcohol dehydrogenase
- Superoxide dismutase
- Transferases like RNA polymerase and aspartate transcarbamoylase
- Hydrolases like carboxypeptidase A and thermolysin
- Lyases like carbonic anhydrase and fructose-1,6-bisphosphate aldolase
- Isomerases like phosphomannose isomerase
- Ligases like pyruvate carboxylase and aminoacyl-tRNA synthases.
- Zinc is not only involved in enzymes, where it plays both a catalytic and a structural role.
- The bioinorganic chemistry of zinc is dominated by a number of factors, the most pertinent of which are summarised here.
- The divalent zinc ion is redox inactive, in contrast, for example, to manganese, iron, and copper.
- Its d10 configuration means that not only does it have no d-d transitions, and therefore no absorption spectroscopy, but also its complexes are not subject to ligand field stabilisation effects such that Zn2+ has no ligand field constraints on its coordination geometry.
- Coordination number and geometry are therefore dictated only by ligand size and charge. This means that zinc can, in principle, adopt highly flexible coordination geometry.
- However, in most zinc proteins, there is a strong preference for tetrahedral coordination, frequently slightly distorted, which enhances both the Lewis acidity of the zinc centre and the acidity of a coordinated water molecule.
Carbonic Anhydrase (Zn metalloenzyme)
Figure 1: Structure of Carbonic Anhydrase
Structure of Carbonic Anhydrase
· The metalloprotein consists of 260 amino acids and contains a Zn2+ ion bound by three His residues in a pocket≈1500 pm deep.
· Molar mass: 30000
· Active site contains Zn2+ ion
Zn2+ ion coordinated tetrahedrally to three histidine imidazole nitrogen atoms and one water molecule.
· Molar mass: 30000
· Active site contains Zn2+ ion
Zn2+ ion coordinated tetrahedrally to three histidine imidazole nitrogen atoms and one water molecule.
- Found in red blood cells, gastric mucosa, pancreatic cells, and renal tubules that catalyzes the inter conversion of carbon dioxide (CO2) and carbonic acid (H2CO3).
- Carbonic anhydrase plays an important role in respiration by influencing CO2 transport in the blood.
- The carbonic anhydrase (CA) form a family of enzymes that catalyze:
H2O +CO2 ——-> H2CO3
- The transport of CO2 around the respiratory system is vital; however the solubility of CO2 in water at physiological conditions is very small.
- Carbonic anhydrase enhances the solubility of CO2 by catalyzing its conversion to the more soluble HCO3– ion.
- In mammals, the HCO3– ion can then be transported to the lungs by the blood stream where it is converted back to CO2 and exhaled.
- The reaction rate of carbonic anhydrase is one of the fastest of all enzymes, and its rate is typically limited by the diffusion rate of its substrates. Typical catalytic rates of the different forms of this enzyme ranging between 104 and 106 reactions per second.
- The reverse reaction is relatively slow in the absence of a catalyst.
- An anhydrase is defined as an enzyme that catalyzes the removal of a water molecule from a compound, and so it is this “reverse” reaction that gives carbonic anhydrase its name, because it removes a water molecule from carbonic acid.
- Enzyme catalyzed reaction rates are pH dependent. The rate of forward and backward reactions in the carbon dioxide equilibrium increases as the pH is raised.
- Existence of a group in the enzyme with a pKa of 7 that must be deprotonated to give the form of enzyme which is required for hydration of CO2.
- An acid for EH+ is required for dehydration in HCO3–, since pH dependence of dehydration is the inverse of that for hydration.
- Activity linked group in the enzyme is an H2O molecule coordinated to Zn2+ ion.
- Presence of basic amino acid side chain, help stabilize the metal bound OH group and enhance its nucleophilicity.
- CO2 is probably hydrogen bonded in order to orient it for nucleophilic attack and to stabilize the negative charge that will build up on the CO2 oxygen atoms.
- The final step would be
- Dissociation of HCO3– away from the active site.
- Removal of H+ from the –BH group by solvent (B = amino acid side chain)
- Coordination of a solvent water molecule to the vacant site on the Zn2+ ion.
Mechanism of action
- The Zn2+ ion is more acidic in carbonic anhydrase than in carboxypeptidase. The presence of a neutral or less basic histidine residue instead of the glutamate residue contributes to the greater acidity of Zn2+ ion.
- The three histidine residues are pulled back; therefore Zn2+ ion becomes more electronegative and more acidic towards the fourth position. Thus, the coordinated water becomes more polarized and losses H+ ion to give Zn-OH–.
- The nucleophilic OH– then attacks on the carbon atom of CO2 captured in the hydrophobic pocket near the Zn2+ ion, and a transient five coordinated Zn2+ ion is formed in which a carbonato oxygen from HCO3– coordinates to the Zn2+ ion.
- After rearrangement, the HCO3– ligand is replaced by H2O. The protonation of H2O coordinated to Zn2+ ion then regenerate Zn-OH– which then attacks another CO2 with the continuation of the catalytic cycle.
Carboxypeptidase A (Zn metalloenzyme)
- Carboxypeptidase A (CPA) is a pancreatic metalloenzyme which catalyses the cleavage of a peptide link in a polypeptide chain during the process of digestion.
Figure 3: Structure of Carboxypeptidase A –
BSc Chemistry Notes and MSc Chemistry Notes of Bioinorganic Chemistry
BSc Chemistry Notes and MSc Chemistry Notes of Bioinorganic Chemistry
- Carboxypeptidase A is produced in the pancreas and is crucial to many processes in the human body to include digestion, post-translational modification of proteins, blood clotting, and reproduction.
- Carboxypeptidase A catalyzes the hydrolysis of peptide bonds of C-terminal residues with aromatic or aliphatic side-chains.
- In addition, there are 4 other mammalian enzymes named CPA-3–CPA-6, and none of these are present in the pancreas. Instead, these other CPA-like enzymes have diverse functions.
- CPA3 is involved in the digestion of proteins.
- CPA4 may be involved in tumor progression, but this enzyme has not been well studied.
- CPA5 has not been well studied.
- CPA6 is present in the extracellular matrix (brain) where it is enzymatically active. A human mutation of CPA-6 has been linked to Duane’s syndrome (abnormal eye movement). Recently, mutations in CPA6 were found to be linked to epilepsy.
Structure of Carboxypeptidase A
• The enzyme consists of a single protein chain of 307 amino acids and one Zn2+.
• Molar mass: 34800
• Zn2+ ion tetrahedrally coordinated to:
Two Histidine N atoms,
An oxygen atom from carboxyl side chain of glutamic residue,
One water molecule.
Chemistry Notes by ChemistryNotesInfo.com
• Molar mass: 34800
• Zn2+ ion tetrahedrally coordinated to:
Two Histidine N atoms,
An oxygen atom from carboxyl side chain of glutamic residue,
One water molecule.
Chemistry Notes by ChemistryNotesInfo.com
- The metal ion is coordinated to two N-atoms histidine residues, to an oxygen atom of a glutamate residue that acts as bidentate ligand and to a water molecule.
- The cavity has a hydrophobic pocket close to Zn2+ ion that can accommodate organic group of the peptide undergoing hydrolysis.
- The site of cleavage is specific in two ways: it occurs at the C-terminal amino acid (Figure 4), and it exhibits a high selectivity for substrates in which the C-terminal amino acid contains a large aliphatic or aromatic side chain.
Figure 4: Reaction of Carboxypeptidase A
- The carbonyl group of the substrate hydrogen bonds to an arginine (Arg-145) and the Zn2+ ion bonds to the oxygen of the peptide carbonyl group (Figure 3).
- The Arg-127 bonds to oxygen of carbonyl group of peptide of substrate and the phenolic group of Try-248 residue hydrogen bonds to-NH group of peptide substrate.
At the active site
- The peptide or protein is bound at the active site by electrostatic attraction between its negatively charged carboxylate ion and arginine-145.
- Zn2+ acts as a Lewis acid toward the carbonyl oxygen, increasing the positive character of the carbonyl carbon.
What happens at the active site?
- Water attacks the carbonyl carbon. Nucleophilic acyl substitution occurs.
- Arg-127 residue forms a strong hydrogen bonded linkage to the terminal carboxyl group as H2O molecule remain in the coordination sphere of the Zn2+ and attacks the substrate by the hydroxo mechanism.
- The Zinc ion serves as lewis acid:
- Binding and polarizing the carbonyl group
- Enhancing the acidity of the bound group so it can become necessary OH nucleophile.
Mechanism of action
Figure 1
• In the first step, the peptide to be cleaved is ‘manoeuvred’ into position close to the Zn2+ site; the dominant substrate–protein interactions involved at this stage are:
Salt-bridge formation between the C-terminal carboxyl ate group of the substrate and residue Arg-145 which is positively charged;
Intermolecular interactions between the non-polar group R’ and residues in a hydrophobic pocket of the protein chain.
• These interactions may be supplemented by hydrogen bond formation between the OH group of Tyr-248 and the N-H group indicated in the figure, and between Arg-127 and the C=O group adjacent to the peptide cleavage site. This latter interaction polarizes the carbonyl group, activating it towards nucleophilic attack.
• In the first step, the peptide to be cleaved is ‘manoeuvred’ into position close to the Zn2+ site; the dominant substrate–protein interactions involved at this stage are:
Salt-bridge formation between the C-terminal carboxyl ate group of the substrate and residue Arg-145 which is positively charged;
Intermolecular interactions between the non-polar group R’ and residues in a hydrophobic pocket of the protein chain.
• These interactions may be supplemented by hydrogen bond formation between the OH group of Tyr-248 and the N-H group indicated in the figure, and between Arg-127 and the C=O group adjacent to the peptide cleavage site. This latter interaction polarizes the carbonyl group, activating it towards nucleophilic attack.
Figure 2
• The nucleophile is the H2O ligand coordinated to Zn2+. The Lewis acidity of the metal ion polarizes the O-H bonds.
• The carboxylate group of Glu-270 assists in the process by removing H+ from the H2O ligand.
Figure 3
• The cleavage of the peptide C-N bond for which H+ is probably provided by Glu-270.
• It appears likely that the second H+ required for the formation of the NH3+ group on the departing terminal amino acid comes from the terminal CO2H group of the remaining portion of the substrate (Figure 4).
Figure 4
• Figure 3 shows Glu-72 bound in a monodentate manner to the Zn2+ centre, whereas in the rest state, a bidentate mode has been confirmed (Figure 1).
• A change from a bi- to monodentate coordination appears to be associated with the formation of the Zn2+—-O—-H(Arg-127) interaction illustrated in Figure 3, the Zn2+ ion being able to move towards Arg- 127 as the interaction develops. To complete the catalytic cycle, an H2O ligand refills the vacant site on the Zn2+ centre.
Liver Alcohol Dehydrogenase (Zn metalloenzyme) – Zinc Metalloenzymes MSc BSc Chemistry Notes
- Group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+ to NADH).
Structure of Liver Alcohol Dehydrogenase
Structure of Liver Alcohol Dehydrogenase
- Liver Alcohol Dehydrogenase is a dimer of two subunits.
- It has two Zn atoms per subunits.
- Two of its four Zinc atoms lie at the catalytic sites.
- It consists catalytically active and inactive Zn2+ ions.
- In humans and many other animals, they serve to break down alcohols that otherwise are toxic, and they also participate in generation of useful aldehyde, ketone, or alcohol groups during biosynthesis of various metabolites.
- In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+.
- They are dimeric proteins, with each subunit binding two Zn2+ ions, only one of which is catalytically active.
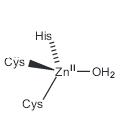
- This catalytic Zn2+ ion has distorted tetrahedral geometry, coordinated to one histidine and two cysteine residues.
- The noncatalytic zinc plays a structural role and is coordinated tetrahedrally to four cysteine residues.
- Enzyme lies in pocket some 20Å from the surface
In-active structure
In-active structure – Regular tetrahedral, coordinated to four cysteine sulfur atoms
Active structure
Active structure – Distorted tetrahedral, coordinated to two cysteine sulfur atoms, one histidine and a water molecule
Mechanism of action
- Enzyme lies in the pocket; due to this it is unlikely that Zinc atoms can form pentacoordinate intermediate.
- After binding of NAD+, the water molecule is displaced from the zinc atom by the incoming alcohol substrate.
- A coenzyme NAD+ binds to the protein with C-4 of its positively charged pyridine ring lying about 4.5Å from the active Zinc.
- Deprotonation of the coordinated alcohol yields a zinc alkoxide intermediate, which then undergoes hydride transfer to NAD+ to give the zinc-bound aldehyde and NADH.
- A water molecule then displaces the aldehyde to regenerate the original catalytic zinc centre, and finally NADH is released to complete the catalytic cycle.
- Thus, the role of zinc in the dehydrogenation reaction is to promote deprotonation of the alcohol, thereby enhancing hydride transfer from the zinc alkoxide intermediate.
- Conversely, in the reverse hydrogenation reaction, its role is to enhance the electrophilicity of the carbonyl carbon atom.
- NAD+ assist in removal of a hydride ion from the µ CH2 group of the metal bound alcohol substrate by forming NADH.
- The essential features of the catalytic cycle are summarised below.
Mechanism of action of Liver Alcohol Dehydrogenase (Zn metalloenzyme) – BSc & MSc Chemistry Notes in Hindi English with PDF by Chemistry Notes Info
Cobalt-for-zinc ion substitution
- A practical disadvantage of working with metalloproteins containing Zn2+ is the d10 configuration of the ion. The metal site cannot be probed by using UV-VIS or EPR spectroscopies or by magnetic measurements. Such methods were especially important before protein crystallography became a widely applied technique.
- Studies involving Co2+ -for-Zn2+ substitution provide a metal centre that is amenable to investigation by spectroscopic and magnetic techniques (Co2+ is a d 7 ion), the choice of Co2+ being because:
- The ionic radii of Co2+ and Zn2+ are about the same;
- Co2+ can tolerate similar coordination environments to Zn2+
- It is often possible to replace Zn2+ in a protein by Co2+ without greatly perturbing the protein conformation.
For BSc and MSc chemistry notes visit below categories
Also Like Share and Comment on these free chemistry notes by Your Chemistry Tutor CHEMISTRY NOTES INFO




















%20(1).png)
