ELECTROCHEMISTRY Class 12 Notes
These are electrochemistry class 12 notes. Dear science students you can also get electrochemistry class 12 notes pdf from download section of Chemistry Notes Info website page.
Electrolysis:
It is the process of decomposition of an electrolyte by the passage of electricity through its aqueous solution or molten state.Faraday’s first law of electrolysis:
In a chemical reaction, the amount of any substance deposited or liberated is directly proportional to the quantity of electricity passed through it.
W
∝ Q
W
= ZQ
W
= Zit ( Q= I*t
)
Where,
Z = Electrochemical Equivalent.
Z = atomic
weight/nF (n = no. of
electron, F = 96500 )
Faraday’s second law of electrolysis:
When the same quantity of electricity is passed through the different electrolytes connected in series. The weights of the substance produced at the electrodes are directly proportional to their equivalent weights.Conductance of electrolytic solution: -
Electrolytes conducts electricity by decomposition.Electrical resistance: -
If voltage v is applied to the ends of the conductor and current “I” floe through it, then the resistance “R” of the conductor is V/I (ohm).Electrical conductance:-
The reciprocal of electrical resistance is called conductance. And is represented by G sign
Thus, G = 1/R [unit of G = mho]
Specific
resistance or Resistivity:- it is observed that,
Specific conductance or conductivity:-
The reciprocal of resistivity is known as conductivity. And is denoted by k (kappa).Equivalent conductivity:-
Equivalent conductivity of a solution at a dilution v is defined as the conductance of all the ions produced from one gram equivalent of the electrolyte dissolved in v cm3 of the solution when the distance between the electrode is one cm and the area of the electrode is so large then whole the solution is come between them.Where, c is molar concentration.
Kohlrausch law: -
The limiting molar conductivity of an electrolyte (i.e. molar conductivity at infinite dilution) is the sum of the limiting ionic conductivities of the cation and the anion each multiplied with the number of ions present in one formula unit of the electrolyte.Kohlrausch law in terms of equivalent conductivity:-
The equivalent conductivity of an electrolyte at infinite dilution is the sum of two values one depends upon cation and other upon anion.Electrode Potential :-
The electrical potential difference set up between metals and its ions in the solution is called electrode potential.
Cell
potential or EMF of a cell:- The difference between
the electrode potential of the two half cell is known as cell potential or cell
voltage it is called Electromotive Force
or EMF of the cell if no current is drawn from the cell.
Electrochemical series:-
The various electrodes have been arranged in order of their increasing value of standard reduction potentials. This arrangement is called electrochemical series.Nernst equation for electrode potential:-
Mn+
+ ne- --> M
Then Nernst equation is,
Where, E = electrode potential under given concentration of Mn+
ions and temperature T.
E0 =
standard electrode potential.
R = gas
constant.
T = Temperature
in K
F = one Faraday
n = No. of
electrons involves in the electrode reaction.
Also put, R = 8.314
Jk-1mol-1
F =
96500 coulombs
Primary Cells
Dry cell:-
It consist of a cylindrical zinc container which acts as a anode. A graphite rod is placed between the center, acts as a cathode. The space between the cathode and the anode is so packed with the paste of NH4Cl and ZnCl2 and graphite is surrounded by MnO2 and carbon.
Reactions,
At Anode: - Zn
(s) -->
Zn2+ (aq) + 2e-
At cathode: - 2MnO2
(s) + 2NH4+ (aq) + 2e- -->
Mn2O3 (s) + 2NH3 (g) +H2O
These cells have voltage in range of 1.25 V to 1.50 V have no
long life.
Mercury cell (Ruber Mallory cell) :-
It consists of zinc container as anode, a carbon rod as cathode and a paste of mercuric oxide mixed with KOH as the electrolyte a lining of porous paper separate the electrolyte from zinc container.
Cell
reaction:-
At
Anode : - Zn + 2OH- --> ZnO + H2O +
2e-
Secondary cell
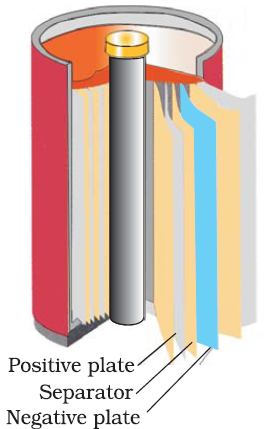
Lead storage battery:-
cell consist of lead anode and a grid of lead packed with lead dioxide acts as lead cathode. These electrodes are arranged alternatively separated by fiber glass sheet and suspended in sulfuric acid (dilute) which acts as electrolyte.Electrode reaction occurs during discharge battery:-
At Anode :-
Pb + SO42- --> PbSO4
+ 2e-
At Cathode :- PbO2 + SO42-
+ 4H+ + 2e- --> PbSO4
+2H2O
In above reactions H2SO4 is
used hence density of H2SO4 is decreases so battery will
discharge.
During charging:-
The electrode reaction is reserved.
PbSO4 + 2e- -->
Pb + SO42-
PbSO4 +2H2O -->
PbO2 + SO42- + 4H+ + 2e-
Nickel Cadmium Storage Cell:-
It consists of cadmium electrode (as Anode) and metal grid of nickel (iv) oxide as cathode immersed in KOH solution.Reaction occurs during discharge of cell:-
At
Anode :- Cd + 2OH- --> Cd(OH)2 + 2e-
At
Cathode :- NiO2 + 2H2O +2e- -->
Ni(OH)2 + 2OH-
Hence,
The reaction can be reversed during charging. The
potential of each Ni-Cd cell is approximately 1.4 V
Fuel cell: -
It consists of porous carbon electrodes containing suitable catalyst incorporated in them. Concentrated KOH or NaOH solution is placed between the electrodes to act as the electrolyte. The hydrogen or oxygen gases are bubbled through the porous electrode into the KOH/NaOH solution.
Reactions:-
At Anode: - 2H2 + 4OH- -->
4H2O + 4e-
At Cathode:
- O2 + 2H2O + 4e- -->
4OH-
CORROSION
Corrosion:-
The process of slowly eating away of metal due to attack of atmospheric gases on the surface of metal resulting into the formation of compounds such as oxides, sulphides, carbonates, sulphates etc. is called as corrosion.
In corrosion the metal is oxidized by loss of
electrons to oxygen and formation of oxides. The corrosion of iron occurs in
the presence of air & water.
In iron sheet or object or a particular pot behaves
like a where oxidation takes place.
Anode: - 2Fe (s) --> 2Fe2+
+ 4e-
And these electron moves
to another spot where reduction takes place in the presence of H+
ion (H2CO3)
At Cathode: - O2(g) + 4H+ (aq) + 4e- -->
2H2O (l)
The overall reaction being
2Fe + O2 + 4H+ --> 2Fe2+ + 2H2O
E0(cell)
= 1.67 v
Fe2+ (ferrous ion) oxidised by O2to form
ferric ion (Fe3+) which comes out as Rust Fe2O3.xH2O.
Dear Science students, you learn electrochemistry, now you can test your knowledge about electro-chemistry with our Electrochemistry Quiz. By learning these notes and solving MCQs quiz you became able to pass examinations by solving all electrochemistry important questions.


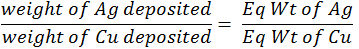

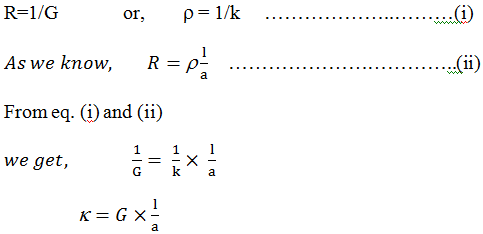

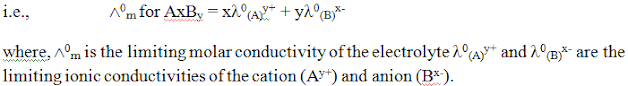









%20(1).png)
