Structure of Atom Class 11 Chemistry Notes Chapter 2
Atomic Theory of matter :-
According to this theory, atom is the ultimate particle of matter. This atomic theory of matter is also known as Dalton’s Atomic theory (1808).Dear learner, if your basics are not clear about structure of atome then please read our post on basics under title Structure of atom class 9 notes. Otherwise continue reading these Structure of atom class 11 notes. If you want to learn in more details, then read our post on Structure of atom BSc chemistry notes.
- Cathode rays start from cathode and move toward anode.
- These rays are not visible but there behavior can be observed with fluorescent or phosphorescent material.
- In the absence of magnetic or electric field these rays travels in straight lines.
- In the presence of magnetic or electric field, the behavior of cathode rays is similar to Negatively charged particles. Which suggest that these rays contain negatively charge particles called electron.
- Cathode rays (or electrons) do not depend on the material of the electrode and nature of the gas in the tube so electrons are basic constituent of all atoms.
Charge (e) to mass (me) ratio of electron
- Measured
by J. J. Thomson (1897).
- By
using cathode ray tube.
- By applying electrical & magnetic field perpendicular
to each other & also perpendicular to path of electrons.
- He
proposed deviation of particles from
their path in presence of magnetic or electrical field depend upon the
following-
1. Magnitude of – ve charge
on particle
i.e.
if magnitude of charge on particles is greater than interaction with magnetic
or electric field is greater so deflection is also grater.
2. Mass of
particles
i.e.
particle is lighter then deflection is greater.
3. Strength of magnetic or electric field.
i.e. if strength of
magnetic field or voltage, at electron is
increases then deflection of e-
also increases.
=> value of e/me =
1.758820× 1011 C kg-1
Charge of electron
= Determine
by R. A. Millikan
= By
oil drop experiment (1906-1914)
= Charge
on e- = -1.6× 10-19
C
= Present
accepted value , e- = -1.6022× 10-19 C
Mass of electron
From charge on e- & e/me
Me = 9.1094
´
10-31 kg
Discovery of protons
= Discovered
by E. Goldstein.
= In
modified cathode ray tube gives +ve charge carrying particles known as canal
rays.
= Lithest
& smallest +ve ion obtained from Hydrogen called proton.
Characteristics
1) Depend
upon, nature of gas present in cathode ray tube.
2) Charge
to mass ratio of particles depends on gas from which these originate.
3) Some
of +ve charged particles carry a multiple unit of electrical charge.
4) Behavior
of protons in magnetic or electric field is opposite to that of electron behavior.
Discovery of neutrons
Discovered
by Chadwick (1932).
By
bombarding a thin sheet of beryllium by alpha particles.
Electrically
neutral particles were emitted known as neutrons.
Thomson model of atom
Given by J.J Thomson (1898)
According
to J.J. Thomson, atoms posses a spherical shape,
with radius about 10-10 m,
in which + ve charge is uniformly distributed.
Electrons
are embedded in such a manner to give most stable electrostatic arrangement.
Other
names of this model plum pudding, raisin pudding, watermelon model.
Mass
is assumed to be uniformly distributed in atom.
Rutherford’s nuclear model of atom
= Given
by Rutherford & his students Ernest Marsden and Hans Geiger.
= When
beam of high energy α- particles was directed at gold foil, then tiny flash of
light observed at photographic plate.
Rutherford observed that-
- 1) Most of the α- practical passed through gold foil un-deflected.
- 2) A small fraction of α- particles was deflected by small angles.
- 3) A very few α- particles (about 1 in 20,000) bounced back means deflected by nearly 1800
From above observations he concludes the structure of atom.
1) Most
of space in atom is empty because most of α- particles passed un-deflected.
2) Few
+ve charged α- particles were deflected.
Because
+ ve charge of the atom present in center in very small volume that repelled
& deflected the +ve charged α- particles.
3) Volume
of nucleus is negligible as compared to total volume of atom
i.e. radius of atom = 10-10m (approx)
radius
of nucleus = 10-15m (approx)
On the basis of observation & conclusion Rutherford proposed model of atom as-
1) +ve
charge & most of mass present in the center of atom known as nucleus.
2) Electrons
moves around nucleus with very high speed in circular paths known as orbits.
3) Electrons
and nucleus (protons) are held together by electrostatic force of attraction .
Atomic
number (Z)
= no of protons in the nucleus of an atom
= no of
electrons in a neutral atom
Mass
number (A) = number
of protons (z) + number of Neutron (n)
Isobars :-
These are atoms with same mass number but different atomic number.Isotopes: -
These are atoms with same atomic number but different atomic mass no.
Wave nature of electromagnetic radiations: -
First explanation given by James Maxwell (1870).
1) Oscillating
magnetic & electric fields produced by the oscillating charged particles
are perpendicular to each other and also perpendicular to the wave
direction of propagation.
2) These
waves do not require medium i.e. electromagnetic wave can travel in vacuum.
3) Electromagnetic
radiation differs from one another in frequency or wavelength gives
electromagnetic spectrum.
4) Different
units are used to represent electromagnetic radiation.
n
= frequency,
l = wavelength.
Particle nature of electromagnetic radiation :-
Also known as Planck’s Quantum theory
= Planck suggested that the atoms and molecules
can absorb or emit energy in discrete quantities not in continuous manner.
Planck gives it name as quantum. Energy (E) of
quantum of radiation is directly
proportional to its frequency(n)
i.e.
E=hn
Where, h =
planks constant = 6.626× 10-34 js
Photo electric effect:-
= given
by H. Hertz (1887)
= When
a beam of light strike a metal surface then electrons were ejected. This phenomena
is known as photo electric effect.
1. Electrons
ejected from metal surface, when beam of
light strike the metal surface.
2. Number
of electron ejected is directly proportional to intensity (or brightness) of
light.
3. There
is characteristic minimum frequency (n0
threshold frequency) below which photoelectric effect is not observed.
4. If
n
> n0
then electrons comes out with kinetic energy which increases with increase in frequency
of light.
Kinetic
energy of ejected electrons is given by-
h
n
= h n0+
½(meV2)
Spectroscopy:-
study of absorption or emission spectra is called spectroscopy .Bohr’s model for hydrogen atom:-
= Explain
by Nails Bohr (1913).
= Postulates
for Bohr’s modal are,
1. Electron
in hydrogen atom move around nucleus in circular path of fixed radius and energy.
these paths are called orbits.
2. Energy
of electron does not change with time.
However, when electron move from
lower to higher stationary state. It absorbed some amount of energy and energy
release when it comes back.
3. Frequency
of radiations emitted or absorbed when transition of electron occur is given by
Limitation of Bohr’s model:-
1. Bohr
model fail to explain fine detail of hydrogen atom spectrum observed by spectroscopic,
techniques.
2. It
fails to explain spectrum of other atom except hydrogen atom.
3. It
fails to explain splitting of the spectral lines in presence of electric field (stark
effect) or magnetic field ( Zeeman effect ).
4. Fail
to explain formation of molecules from atoms by chemical bonding.
Dual behaviour of matter :-
= Explain
by de-Broglie (1924)
= He
explain that matter also behave like radiation and exhibit dual behavior means
both like particle and wave like properties .
= Relation
m = mass of particle ,
v = velocity of particle,
p =
momentum
Heisenberg’s uncertainty principle:-
Given by Werner Heisenberg (1927)
He explain that it is impossible to
determine simultaneously the exact position and exact momentum (or velocity) of
an electron.
Mathematical explanation
Where, Dx= uncertainty in position
DVx = uncertainty in velocity or momentum
Quantum mechanical model of atom:-
- Branches of science which explain duel behavior of Metter is called quantum mechanics.
- Quantum mechanics independently developed by Werner Heisenberg and Erwin Schrodinger (1926).
- Fundamental equation developed by Schrodinger. (He won Nobel Prize in 1933).
- Equation for a system (atom or molecules was, energy does not change with time)
Principle quantum number ‘n’ :-
·
It is a positive Integer with value of n
= 1,2,3......
·
It determine size and energy of orbital.
·
It also identifies the shell with
increase in number of allowed orbital.
And given by n2
N =1,
2, 3, 4........
Shell
= k, l, m,
l......
·
Size of orbital increase with increase in n.
Azimuthal quantum no.‘l’ :-
·
It is also known as orbital angular
momentum or subsidiary quantum no.
·
It defined 3D shape of orbital.
·
For given value of n possible value
of
L= 0,1,2,3,4,5,----------(n-1) ,
Ex :- if n=1 then
l=0
if n=2
then l=0,1
if n=5
then l=0,2,3,4
·
Each shell consists of one or more
sub-shells.
·
No of sub-shells = value of n
If n= 1
then 1 sub-shell = (l=0)
If n= 2
then 2 sub-shell = (l=0,1)
If n= 3
then 3 sub-shell = (l=0,1,2)
·
Value of l =
0, 1, 2,
3, 4, 5 ----------
Notation for sub-shell= s, p, d, f, g,
h--------------
·
Sub-shell notation
n
|
l
|
Sub-shell
notation
|
1
|
0
|
1s
|
2
|
0
|
2s
|
2
|
1
|
2p
|
3
|
0
|
3s
|
3
|
1
|
3p
|
3
|
2
|
3d
|
4
|
0
|
4s
|
1
|
4p
|
|
4
|
2
|
4d
|
4
|
3
|
4f
|
Magnetic orbital quantum no ‘mi’ :-
·
This quantum no (mi) gives information
about orientation of the orbital .
· mi = (2l+1) i.e. if value of l
is 1 then value of mi = 2×1+1=3=(-1,0,1)
Value of p
|
0
|
1
|
2
|
3
|
4
|
5
|
Sub-shell
notation
|
S
|
P
|
D
|
F
|
G
|
H
|
No of orbital’s
|
1
|
3
|
5
|
7
|
9
|
11
|
Electron spin quantum (ms) :-
·
Proposed by G. Uhlen Beck & S.
Goodsmit (1925).
·
Electrons spins around its own axis.
·
Ms have two value +1/2 &
-1/2.
·
Ms gives information about
orientation of the spin of the
electron.
Aufbau principle :-
According to this principle, in the ground state of the atoms the orbital’s are filled in order of their increasing energies. Means electrons enter higher energy orbital’s, so order in which orbital’s are filled is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.Pauli exclusion principle :-
·
Given by W. Pauli (1926).
·
Two electrons in an atoms can’t have
same set of 4-quantum no.
·
Only two electrons may exist in same orbital
and these electrons must have opposite spin.
Hund’s rule of maximum multiplicity :-.
·
According to this rule, pairing of electron in the orbital’s belonging to the
same sub-shell (p, d or f) does not take place until each orbital
belonging to that sub-shell has got one electron each i.e. it is singly occupied.
These Chemistry notes are published by Chemistry Notes Info website www.ChemistryNotesInfo.com
Structure of Atom Class 11 MCQ
1. Who discovered anode rays :
GoldsteinRutherford
J. Stanley
J. J. Thomson
2. Neutron was discovered by :
RutherfordChadwick
Austin
Langmuir
An electric field only
By None
Read More...
3. Radioactive isotope of hydrogen has ________ number of neutrons :
0
1
2
3
4. Cathode rays are deflected by :
A magnetic field onlyAn electric field only
By None
Read More...





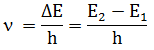


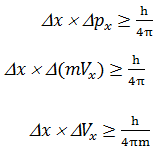
%20(1).png)

Chemistry Apps
ReplyDeleteChemistry Android Apps for 9th, 10th, 11th, 12th, BSc, MSc and Spectroscopy
https://apkgk.com/com.wChemistryNotesInfo
This note is helpful for neet aspirants as well board exam
ReplyDelete