Chemical Kinetics
The branch
of chemistry, which deals with the study of reaction rates and their mechanism,
called as chemical kinetics.
Rate of a chemical reaction:-
“ The rate of a reaction can be defined as the change in concentration of a reactant or product in unit time”
Let a
reaction whose volume remain constant R--->P
One mole of
reactant R produces one mole of
product P. [R1] & [P1]
and [R2] & [P2] are the concentrations of R & P
at time t1 & t2 respectively.
Both above expression
show average rate of reaction
Units of rate of reaction:-
1. Concentration time-1
2. Mol L-1s-1
Instantaneous rate of reaction:-
It is the rate of change of concentration (i.e. change of concentration per unit time) of any one of the reactants or products at that particular instant of time.Factors influencing Rate of a reaction:-
1. Concentration:-
As concentration of reactant increase, rate of reaction also increases.2. Temperature:-
Rate of reaction increases with increase of temperature mostly reaction rate double with rise of 100 temperature.3. Catalyst :-
Catalyst generally increase the rate of reaction without undergoing in the reaction, it also help in attaining the equilibrium quickly without disturbing the equilibrium state in reversible reaction.Rate expression and rate constant:-
Consider a general reaction aA + bB --> cC + dD
Where, a, b, c, and
d are stoichiometric coefficient of reactants and products.
The rate expression for this reaction is-
Rate is directly proportional to [A]x [B]y ………………………..(iii)
Where, component x
& y may or may not be equal to the stoichiometric coefficient (a & b)
of the reactants
Also, Rate = k
[A]x[B]y
………………………………(iv)
This form of equation (v) is known as differential rate
equation, where k is proportionality constant called rate constant. And the
equation (iii) which relates the rate of a reaction to concentration of
reactants is called Rate law or rate
expression.
Rate:-
Rate law is the expression in which
reaction rate is given in terms of molar concentration of reactants with each
terms raised to some power, which may or may not be same as the stoichiometric
coefficient of the reacting species in a balance chemical reaction.
EX:-
Reaction --> Experimental rate expression
CHCl3 + Cl2 --> CCl4 + HCl
Rate = k[CHCl3]
[Cl2]1/2
CH3COOC2H5
+ H2O --> CH3COOH + C2H5OH
Rate = k[CH3COOC2H5]1
[H2O]0
2NO + O2 --> 2NO2
Order of a reaction:-
The sum of powers of the concentration of the reactants in the rate of low expression is called as the order of that chemical reaction.
Rate = k [A]x[B]y
Order = x+y
Order of
reaction may be 0, 1, 2, 3 or even in fraction, Zero order reaction is
independent of concentration. To Download Chemistry Notes in PDF join our Telegram Channel (Search @ChemistryNotesInfo on Telegram App).
Unit of rate constant (k):-
aA + bB --> cC + dD
Rate = k [A]x[B]y
Molecularity of a reaction: -
The no. of reacting species (atoms, ions, molecules) taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reaction is called molecularity of a reaction.Integral rate equation: -
1. Zero order reaction: -
consider a reaction-
d[R] = -k dT
Integrating
both sides,
[R] = -kt + c
……………………….(1)
Where,
c is constant of integration at t = 0, the concentration of reactant R=[R]0
Where,
[R]0 is initial concentration of reactant.
Substitute
in equation (1),
[R]0 = -k ´ 0 + c
[R]0 = c
………………………………(2)
From
equation (1) & (2),
[R] = -kt + [R]0
Kt = [R]0 – [R]
Also,
Rate = k[NH3]0 = k
2. First order reaction: -
consider a reaction-Integrating this equation, we get
ln[R] = -kt + c ……………………………….(1) [where c is constant]
At
t=0, R=[R]0 where [R]0 is the initial concentration of
the reactant
Put
these values in equation (1), we get
ln[R]0 = -k x 0 + c
ln[R]0 = c
……………………..….(2)
From
equation (1) & (2)
ln[R] = -kt + ln[R]0 ……….…….…(3)
kt = ln [R]0 – ln[R]
k = 1/t[ln([R]0 /[R]) ………………………..(4)
At
time t1 from equation (3)
ln[R]1 = -kt1
+ ln[R]0 ………………………...(5)
At
time t2,
ln[R]2 = -kt2
+ ln[R]0 ……………………………(6)
Where,
[R]1 & [R]2 are the
concentration of the reactant at time t1 & t2
respectively
Subtracting
equation (5) from equation (6), we get
Graph:
Half life of a reaction: -
Temperature dependence of the rate of a reaction: -
Most of chemical reactions are accelerated by increase in temperature. It has been found that for a chemical reaction with rise in temperature by 10°, the rate constant is nearly double.
The temperature
dependence of a chemical reaction can be accurately explained by Arrhenius
equation-
k = A e-Ea/RT …………………………(1)
Where, A is Arrhenius
factor or frequency factor
R is gas constant
Ea is activation energy
measured in joules/mole (jmol-1)
Also, in this reaction-
H2 (g) + I2 (g) --> 2HI (g)
According to Arrhenius, This reaction can take place only when a molecule of hydrogen and molecule of iodine collide to form an unstable intermediate. It exist for a very short time and then break up to form two molecule of hydrogen iodide.
The energy required to
form this intermediate, called ‘activation complex’ (C), is known as activation
energy (Ea).
Fig: A plot between ‘ln k’ vs ‘1/T’
In figure, slope =
-Ea/R and Intercept = ln A
So, we can calculate Ea
and A using these values.
Since A is constant for
a given reaction k1 and k2 are the value of rate constant
at temp. T1 and T2 respectively.
Substrate eq. (3) &
(4)
Effect of catalyst:-
A catalyst which alters the rate a reaction without itself undergoing any permanent chemical change.
The action of a
catalyst can be explained by intermediate complex theory.
According to this
theory, A catalyst participate in a chemical reaction by forming temporary
bonds with the reactants resulting in an intermediate complex and decompose to
yield products and catalyst.
R + C --> R-C --> P + C
Reactant + catalyst --> intermediate complex --> product + catalyst
Collision theory of chemical reactions:-
According to this theory “The reactant molecules are assume to be hard spheres and reaction is postulated to occur when molecule collide with each other” “The no. of collisions per second per
unit volume of the reaction mixture is known as collision frequency (Z)”
Another factor which affects the rate of
a chemical reaction is activation energy for a bimolecular elementary reaction.
A
+ B --> Product
Rate
of reaction can be expressed as
Rate = ZAB e-Ea/RT
Where, ZAB
represents the collision frequency of the reactants, A & B and
e-Ea/RT represents the fraction of molecules
with energies equal to or greater than Ea.
= All collision do not lead to the formation of product. the collision in which molecule collide with sufficient kinetic energy (Threshold energy) and proper orientation, so as to facilitate breaking of bonds between reacting species and formation of new bonds to form products are called as effective collision.
Note:- Threshold energy = Activation energy +
Energy possessed by reacting species.
EX:- Formation of methanol from bromoethane
CH3Br + OH- --> CH3OH + Br-
Thanks for Learning ! Download These Notes in PDF Format
















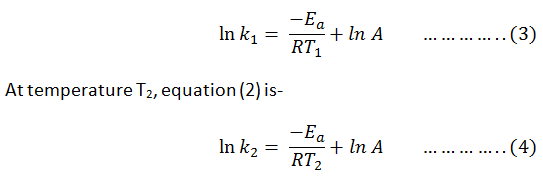


%20(1).png)
