Surface Chemistry
Adsorption: -
The accumulation of molecular species at the surface rather than bulk of solid or liquid is called Adsorption.Adsorbate: -
The molecular species or substance which accumulate at the surface.Adsorbent: -
The surface of material on which Adsorption takes place.Desorption: -
The process of removing of adsorbed substance from the surface on which it is adsorbed.Absorption: -
When the molecular species or substance enters in the bulk phase in solid or liquid is called as Absorption.Sorption: -
When both adsorption and absorption takes place simultaneously is called as sorption process.Mechanism of Adsorption
Inside the
Adsorbent (in bulk) the force acting between the particles are mutually
balanced but on the surface, the particles are not surrounded by atoms or
molecules of their kind on all sides and hence they posses attraction force so
particle stick on the surface of the Adsorbent.
The extent of
adsorption increases with increase in surface area per unit mass of the
adsorbent at a given temperature and pressure.
Heat of adsorption: - With increase in heat Adsorption process
decreases.
Adsorption equilibrium: - As the molecules of the adsorb ate are held
on the surface of the solid adsorbent.
For the process of
adsorption to occur,  G must be negative which is possible only
when,
G must be negative which is possible only
when,  S keeps on decreasing and T
S keeps on decreasing and T S keeps on increasing till ultimately
S keeps on increasing till ultimately  H
becomes equal.
H
becomes equal.
 G must be negative which is possible only
when,
G must be negative which is possible only
when,  S keeps on decreasing and T
S keeps on decreasing and T S keeps on increasing till ultimately
S keeps on increasing till ultimately  H
becomes equal.
H
becomes equal.
To T S so
that
S so
that  G = 0, this state is called adsorption equilibrium.
G = 0, this state is called adsorption equilibrium.
 S so
that
S so
that  G = 0, this state is called adsorption equilibrium.
G = 0, this state is called adsorption equilibrium.Types of adsorption
There are two types
of adsorption
i.
Physical Adsorption or physisorption: - If accumulation of gas on the surface of
solid occurs on account of weak vanderwalls forces is called physical
Adsorption.
ii.
Chemical Adsorption or chemosorption: - When gas molecules or atoms are held to the
surface (solid) by chemical bonds, the Adsorption is called Chemical
Adsorption.
Characteristics of physical Adsorption or physisorption
1) Lack
of specificity: - A given
surface of an Adsorbent does not show any preference for a particular gas as
the vanderwalls forces are universal.
2) Nature
of Adsorbate: - The
amount of gas Adsorbed by a solid depends on the nature of the gas.
3) Reversible
nature: - Physisorption is
reversible because adsorbate may be removed by decreasing pressure.
4) Surface
area of Adsorbent: -
Physisorption increases with increase in surface area.
5) Enthalpy
of Adsorption: -
Physical Adsorption is exothermic process but its enthalpy of adsorption is low
(20-40 KJ mol-1).
Characteristics of Chemical Adsorption or chemosorption
1) High
specificity: - It is
high specific because it occurs if there is some possibility of chemical
bonding.
2) Irreversibility: - As chemisorptions involve compound
formation, so it is usually irreversible process.
3) Temperature: - Chemisorptions increases with increase in
temperature after saturation starts decreasing.
4) Pressure: - it is also increases with increase in
pressure.
5) Surface
area: - chemisorptions
increases with increase in surface area.
6) Enthalpy
of Adsorption: -
Enthalpy of chemisorptions is high (80-240 KJ mol-1) as it involves
chemical bond formation.
Adsorption isotherm: -
The variation in the amount of gas Adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as Adsorption isotherm.Freundlich Adsorption Isotherm: -
Freundlich Adsorption Isotherm is given by freundlich in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature.
Where ‘x’ is the mass of gas
adsorbed on the ‘m’ mass of adsorbent at pressure p, k & n are constant
which depend on the nature of the adsorbent and the gas at a particular
temperature.
Relationship given by curve:-
 |
| Freundlich Adsorption isotherm |
The validity of freundlich
isotherm can be verified by plotting ‘log x/m’ on Y- axis and ‘log P’ on X – axis
it comes to be a straight line.
The adsorption varies directly
with pressure.
Adsorption from solution phase: -
solid can adsorb solutes from solution also.
Example:- litmus solution when
shaken with charcoal becomes colourless.
Factors affecting Adsorption from solution phase
a)
The
extent of Adsorption decreases with increase of temp.
b)
The
extent of adsorption increase with an increase of surface area of the
adsorbent.
c)
The extent
of the adsorption depends upon the concentration of the solute in the solution.
d)
The extent
of Adsorption depends upon the nature of the adsorbent and the adsorbate.
Applications of Adsorption
1) Production of high vacuum
2) Gas masks
3) Control of humidity
4) Removal of coloring matter from solution
5) Separation of inert gases
6) Froth floatation process
7) Chromatographic analysis
Catalysis:-
Berzelius suggested the term catalyst, substance which alter (change) the rate of a chemical reaction and themselves remain chemically and quantitatively unchanged after the reaction are known as catalyst and the phenomenon is known as catalysis.Promoter: -
substance that enhance (increase) the activity of catalyst.Poisons: -
it decreases the activity of catalyst.Homogeneous Catalysis: -
When the reactants and the catalyst are in the same phase (i.e. liquid or gas).Heterogeneous catalysis: -
The catalytic process in which the reactant and the catalyst are in different phase is known as heterogeneous catalyst.Shape Selectivity catalysis by Zeolite: -
The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called as Shape Selectivity catalysis.
“Zeolite” is good shape selective catalysts because of their honey comb like
structure. They are micro porous aluminosilicates with three dimensional networks
of silicates in which some silicone atoms is replaced by aluminium atoms giving
AL-O-Si framework.
An important Zeolite catalyst
used in petroleum industry is ZSM-S.
ZSM-S converts directly
Alcohols into Gasoline (Petrol) by dehydrating them to give a mixture of
hydrocarbons.
Enzyme Catalyst: -
Enzymes are complex nitrogenous organic compound which are produced by living plants and animals work as a catalyst in many life process termed as Biochemical Catalysts (enzyme) and the phenomenon is known as Biochemical Catalysis.Characteristics of Enzyme Catalysis:-
a) Most highly efficient: - One molecule of
enzyme may transform one million molecule of reactant per minute.
b) Highly specific nature: - Each enzyme is
specific for a given reaction.
c) Highly active under optimum temperature: - The
rate of an enzyme reaction is maximum at definite temperature called optimum
temperature (298K-310K).
d) Highly active under optimum PH :- Rate of
enzyme reaction is maximum at optimum
PH (5-7)
e)
Increasing
activity in presence of activators and co-enzymes:- The enzymatic activity in
presence of certain substance called co-enzymes (vitamins) and activators are
generally Na+, CO2+, Mn2+, Cu2+,
etc.
f) Inhibitors
and poisons decrease or stop the rate of enzyme reaction.
Mechanism of Enzyme Catalysis: -
There are number of cavities present on the surface of colloidal particles of enzymes. the molecules of the reactant (substrate ), which have complementary shape ,fit into the theses cavities just like a key fits into a lock .on the account of the presence of actives groups .an Activated complex is formed which then decompose as to yield the products .Colloidal: -
A colloidal is a heterogeneous system in which one
substance is dispersed (dispersed phase) as very fine particles in another
substance called dispersion medium.
Colloidal particles are larger than simple molecules but smaller enough
to remains suspended .their range of diameter is between 1and 1000nm (10-9
to 10-6m)
Classification of collides: -
On the basis of (1) Physical state of dispersed phase and dispersion medium
(2) Nature of interaction between dispersed phase
and dispersion medium.
(3)
Types of particles of dispersed phase.
I.
Classification based on physical state of
dispersed phase and dispersion medium:-
Dispersed
phase
|
Dispersion
medium
|
Types
of colloids
|
Example
|
Solid
|
Solid
|
Solid
sol
|
Some
coloured glasses
|
Solid
|
Liquid
|
Sol
|
Paints
|
Solid
|
Gas
|
Aerosol
|
Smoke,
dust
|
Liquid
|
Solid
|
Gel
|
Cheese,
jellies
|
Liquid
|
Liquid
|
Emulsion
|
Milk,
Hair cream
|
Liquid
|
Gas
|
Aerosol
|
Fog,
mist
|
Gas
|
Solid
|
Solid
sol
|
Pumice,
Stone
|
Gas
|
Liquid
|
Foam
|
Froth,
Whipped cream
|
II.
classification
based on nature of interaction between dispersed phase and dispersion medium :-
in it colloidal state soles
are divided into two categories :- lyophillic (solvent attractive) ,
Lyophobic (solvent repelling)
a) Lyophillic colloids: -
The word ‘lyophillic’ means liquid loving. Colloidal sols directly formed by mixing substances like gum, gelatine, starch, rubber etc. With a suitable liquid (dispersion medium) are called lyophillic sol. These sols are also called reversible sols.b) Lyophobic Colloids: -
These words ‘Lyophobic’ means liquid hating substance like metals their sulphides etc. When simply mixed with the dispersion medium do not form the colloidal sol. Their colloidal sols can be prepared by only special methods; such sols are called lyophobic sols. These sols are also called irreversible sols.
III.
Classification
based on type of particle of dispersed phase:- In it, colloid are classified as
multi-molecular, macro-molecular and associated colloids.
a) Multimolecular Colloids :-
on dissolution, a large no of substance aggregate together to form species having size in the colloidal range (diameter <1nm) the species thus formed are called Multimolecular colloidsb) Macromolecular Colloids :-
macromolecules in suitable solvents form solution in which the size of macro molecules may be in colloidal range .such systems are called macromolecule colloidsc) Associated Colloids (Micelles):-
these are some substances which at low concentration behave as a normal strong electrolyte, but at higher concentration exhibit colloidal behaviours due to the formation of aggregates, the aggregates particle thus formed are called micelles or associated colloids .the formation of micelles takes place only above a particular temperature called Kraft temperature, and above a particular concentration called critical concentration (CMC).Mechanism of Micelles formation :-
soap is the sodium or potassium salt of higher fatty acid and may be represented as RCOO- Na (e.g. sodium stearate , (CH3(CH2)16COO-Na+]) when dissolve into RCOO- and Na+ ions ,the RCOO- ions ,consist of two parts – a long hydrocarbon chain (also called non – polar tail) which is hydrophobic (water repelling ) and a polar group COO- (polar head) which is hydrophilic (water loving ).
But,
At
critical micelle concentration the
anions are pulled into the bulk of the solution and aggregate to form a
spherical shape with their hydrocarbon chain pointing towards the center of the
sphere with COO- part remaining outward on the surface of the
sphere .
Cleansing action of soaps :- the
soap molecule in such a way that hydrophobic part of the stearate ions is in
oil droplet and hydrophobic part projects out of the grease droplet like the
bristles (hairs)
Since the polar groups can interact with water, the oil droplet surrounded by
stearate ions is now pulled in water and removed from the dirty surface thus
soap help in emulsification and washing away of oils and fats
Preparation of Colloids:-
a) Chemical method: -
colloidal solution can be prepared by chemical reaction leading to formation of molecules by double decomposition, oxidation, reduction or hydrolysis. These molecules then aggregate leading to form sols.b) Electrical disintegration or Bredig’s Arc Method: -
this process involves dispersion well as condensation. Colloidal sol of metals such as gold silver etc can be prepared by this method. in this method electric arc is struck between electrodes of metal immersed in the dispersion medium the intense heat product vaporised the metal, which then condenses to form particles of colloidal size.c) Peptization: -
it is defined as the “process of converting a precipitate into colloidal sol” by shaking it with dispersion medium in the presence of a small amount of electrolyte. The electrolyte used for this purpose is called peptizing agent. During peptization: - the particulate absorbs the one of the ions of the electrolyte on its surface. This cause the development of +ve charge on precipitate, which ultimately break up into small particles of the size of a colloid.Purification of colloidal Solution: -
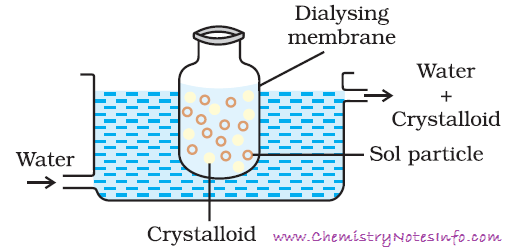
The process used for reducing the amount of
impurities to a requisite minimum is known as purification of colloidal
solution it is carried out by following methods
(I) Dialysis: -
It is a process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane.Since, particles (ions or smaller molecular) in a true solution can pass through an animal membrane (bladder) or parchment paper or colloidal particles. The molecules and ions diffuse through membrane into the outer water and pure colloidal solution to left behind.(II) Electro dialysis: -
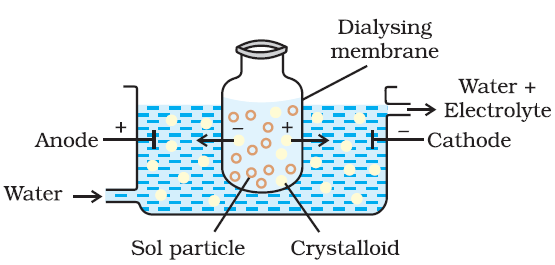
The process of dialysis is quite slow it can be made faster by applying an electric field electrodes are fitted in the compartment. The ions present in the colloidal solution migrate out to the oppositely charge to electrodes.(III) Ultra filtration: -
In these methods special filters are used, which are permeable to all substances except the colloidal particles. An ultra filter paper may be prepared by soaking the filter paper in a colloidal solution, hardening by formaldehyde and then finally drying it. Thus, by using ultra filter paper the colloidal particles are separated from rest of the materials. The colloidal particles left on the ultra filter paper are there stirred with fresh dispersion medium (solvent) to get a pure colloidal solution.Properties of colloidal solutions
(I) Colligative Properties: -
The values of colligative properties (osmotic pressure, lowering in vapor pressure, depression in freezing point, elevation in boiling point) are of small order as compared to values shown by true solution at same concentration.(II) Tyndall effect: -
It is may be defending as the scattering of the light by the colloidal particles present in the colloidal solution.(III) Colour: -
The colour of the colloidal solution depends on the wavelength of the light scatter by the dispersed particles. The wavelength of light further depends on the size and nature of the particles.(IV) Brownian Movement: -
It may be defined as continuous zigzag movement of the colloidal particles in a colloidal solution is known as Brownian movement.(V) Charge as colloidal particles:-
Charge as colloidal particles always carry is electric charge. The nature of this charge is the same on all the particles in a given colloidal solution and may be either +ve or –ve.
Example:-
(a) +ve charged solution:-
1. Haemoglobin
2. Hydrated
Metallic oxides
E.g.
AS2S3.xH2O
(b) –ve charged solution:-
1. Metals
E.g.
Cu, Ag, Au
2. Metallic
sulphides
E.g.
AS2S3, Sb2S3, CdS solutions.
(VI) Electrophoresis: -
The movement of colloidal particles under the influence of an electric field is called electrophoresis. –ve charged particles move towards the cathode and +ve charged particles moves towards anode.Coagulation: -
The process of setting of colloidal particles is called coagulation or precipitation of the sol.
The
coagulation of the lyophobic sols can be carried out in the following ways:-
1. By electrophoresis: - The
colloidal particles move towards oppositely changed electrodes get discharged
and precipitate.
2. By mixing two
oppositely charged sols: - Oppositely charged sols when
mixed almost equal proportional neutralise then changed and get partially or
completely precipitated.
3. By Boiling:
- When a sol is boiled the adsorbed layer is disturbed due to increased
collisions with the molecules of the dispersion medium. This reduces the charge
on the particles and ultimately led to setting down in the form of a
precipitate.
4. By Persistent dialysis:
- On prolonged dialysis, traces of the electrolyte present in the sol are
removed almost completely collides become unstable and ultimately coagulate.
5. By addition of
electrolyte : -
When excess of electrolyte is added the colloidal particles
precipitated, the reason is that colloids interact with ions carrying change
opposite to that present on themselves, this causes neutralisation leading to
their coagulation.
Coagulation of lyophilic sols: -
There are two factors which are responsible for stability or the lyophilic sols. These factors are change and salvation of the colloidal particles. When these two factors are removed, a lyophilic sol can be coagulated. This is done,
(i) By
addition of an electrolyte
(ii) By
adding a suitable solvent
Protection of colloids:-
Lyophilic sols are more stable than lyophobic sols.
Lyophilic colloids have a unique property of protecting lyophobic colloids.
When a lyophilic sol is added to lyophobic sol, the lyophilic particles
(colloids) covering up the particles of lyophobic sol.
Emulsions:-
An emulsion is a colloidal dispersion in which both
the dispersion medium and dispersed phase are liquids generally; one of the two
liquids is water. There are two types of emulsions.
1. Oil
dispersed in water (o/w type) and
2. Water
dispersed in oil (w/o type)
1. O/W type
– water act as a dispersion medium.
Example: -
Milk and vanishing cream.
2. W/O type
- oil act as dispersion medium.
Example: - Butter and cream.
Colloids around us: -
Most of the substances all we come across in our daily life are colloids, for example meals, clothes, wooden, furniture, houses, newspaper are largely composed of colloids.Application of colloids: -
colloids are widely used in the industrial sector.
Example:-
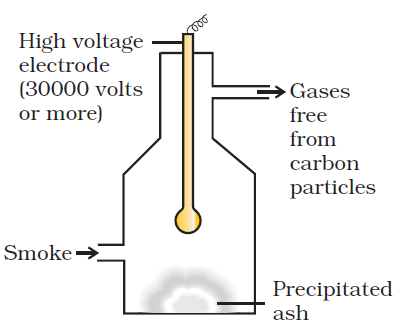
· Electro precipitation of smoke: -
The smoke, before is comes out from the chimney, is led through a chamber containing plates having a charged opposite to that carried by smoke particles. The particles on coming in contact with these plates lose their charge get precipitated, the particles thus settle down on the floor of the chamber. The precipitator is called Cottrell precipitator.· Purification drinking water: -
alum is added to water (that contain impurities) to coagulate the suspended impurities make water fit for colloidal in nature.
Example: - Argyrol is silver sol is
used as an eye lotion.







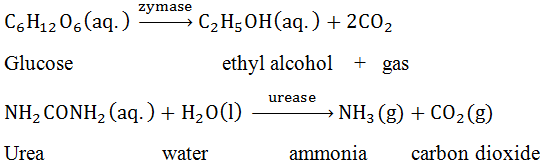






%20(1).png)
