Discovery of Electron
Sir J.J.Thomson and W.Crooks did many experiments with discharge tube for the discovery of electron.
Discharge tube have tube like shape made from glass with two electrodes
(Cathode -ve and Anode +ve) in vacuum created by vacuum pump connected
to discharge tube. High electric potential is applied between two
electrodes.
Air is bad conductor of electricity so vacuum pump is connected to
reduce pressure to 0.02mm inside discharge tube currents starts flowing
between electrodes and light is emitted. On further reducing pressure in
discharge tube greenish yellow color fluorescence occur. As these rays
emerging from cathode, Sir J.J.Thomson named them as cathode rays.
Deflection of cathode rays towards positively charged plate in electric field proves that these rays carry negatively charged particles.
These negatively charged particles are named as electrons.
 | |
| Discharge Tube |
Deflection of cathode rays towards positively charged plate in electric field proves that these rays carry negatively charged particles.
These negatively charged particles are named as electrons.
Properties of Cathode Rays
1. Cathode rays always travel in straight line. |
| Production of Cathode Rays |
3. On applying electric field in the path of cathode rays, cathode rays turn towards +vely charged plate that proves cathode rays are made up from negatively charged particles.
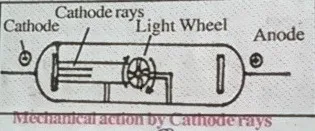
4. Cathode rays rotate light wheel placed in their path that proves cathode rays are made from particles having mass.
6. These rays produce fluorescence at walls of glass tube.
7. Cathode rays ionize gases and also affect photographic plate.
8. When these rays strike any metal with high melting point (like tungsten W) they produces X-Rays.


%20(1).png)
