Physical states of matter
=> Solid, liquid, gas.
Physical
state
|
Definite
volume
|
Definite
shape
|
Example
|
Solid
|
Yes
|
Yes
|
Book, pen
|
Liquid
|
Yes
|
No
|
Water, milk
|
Gas
|
No
|
No
|
CO2, Air
|
Classification of matter: -
1. Mixture:
a.
Homogeneous mixture
b.
Heterogeneous mixture
2. Pure substance:
a.
Elements
b.
Compounds
1. Mixture: - It contains two or more
substances in any ration known as its components.
Ex: - air, tea etc.
a.
Homogeneous mixture:- its components are
completely mix with each other and have uniform composition throughout the
solution.
Ex:- sugar solution, air.
b.
Heterogeneous mixture:- its components do not
have uniform composition throughout solution sometimes different components
observed.
Ex:- mixture of salt & sugar, sand & soil.
2. Pure substances:- these have fixed
composition.
Ex:- copper, gold, water etc.
a. Elements:- It consists of only one type
of particles (i.e. atoms or
molecules)
Ex:- H2, O2, Au, Ag.
b. Compounds:- It consist of two or more
atoms to give a molecule of compound
Ex:- CO2, NH3, H2O.
Properties of matter
1) Physical properties:- Those properties
which can be measured or observed.
2) Chemical properties:- those properties
in which chemical changes occurs.
Ex:-
Acidity, Basicity, Combustibility etc.
SI :- International System of units
=>
Established by 11th general
conference on weight and measures.
=>
Si have seven fundamental units
=>
Other units are divided from seven fundamental
units
Mass:- amount of matter in object
=>
1 kg = 1000 gram
Weight: -force on the object by gravity.
Volume:- (length)3
=>
1 L = 1000 ml, 1000 cm3 = 1dm3
Density:- Amount of mass per unit volume.
=>
SI unit :- Kgm-3
Temperature:- It is the measurement of cooling or warming.
Three scales
=>
0C (Degree Celsius)
=>
0F degree Fahrenheit)
=>
k (Kelvin)
=>
freezing point of water = 0 0C
=>
Boiling point of water = 100 0C
Relationship
Or,
k = 0C +
273.15
Precision:- Closeness
of various measurement for same quantity.
Accuracy:- It is the
agreement of a particular value to the true value of the result.
Significant figure:-
The meaningful digits which are known with certainty.


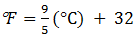
%20(1).png)
