What are the factors which favour formation of Ionic Bonds?
These are the factors which favours formation of ionic bonds...
1. One of the atoms i.e. Metal atom must have low ionization energy, so that it can easily lose its electrons.
2. Other atoms i.e. Non-Metal atoms must have high electron affinity, so that it can hold the extra electrons.
3. One of the atoms i.e. Metal atom should be large in size.
4. Other atoms i.e. Non-Metal atoms should be small in size.
5. Lattice energy of the crystal should be high, means the electrostatic attraction between charged ions in the crystal should be high.
6. Anion and cation should have inert gas electronic configuration.
7. The combining elements should differ by at least 1.9 in electronegativity.

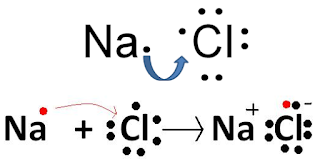
%20(1).png)
