Dual Behaviour of Matter
=> Explain
by de Broglie (1924)
=> He
explain that matter also behave like radiation and exhibit dual behavior means
both like particle and wave like properties .
=> Relation
m = mass of particle ,
v = velocity of particle,
p =
momentum
Heisenberg’s Uncertainty Principle
Given by Werner Heisenberg (1927)
He explain that it is impossible to
determine simultaneously the exact positive and exact momentum (or velocity) of
an electron
Mathematical explanation
Where, Dx= uncertainty in position


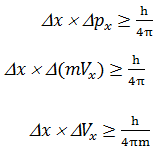
%20(1).png)
