Bohr’s model for hydrogen atom
=> Explain
by nails Bohr (1913).
=> Postulates
for Bohr’s modal are,
1. Electron
in hydrogen atom move around nucleus in circular path of fixed radius and energy.
these paths are called orbits
2. Energy
of e does not change with time.
However, when electron move from
lower to higher stationary state it absorbed sub amount of energy and energy
release when it comes back.
3. Frequency
of radiations emitted or absorbed when transition of e occur is given by
Limitation of Bohr’s model
1. Bohr
model fail to explain finer detail of hydrogen atom spectrum observed by spectroscopic,
techniques.
2. It
fails to explain spectrum of other atom except hydrogen atom.
3. It
fails to explain splitting of the spectral lines in presence of electric (stark
effect) or magnetic field ( Zeeman effect )
4. Fell
to explain formation of molecules from atoms by chemical bonding.

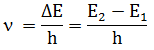

%20(1).png)
