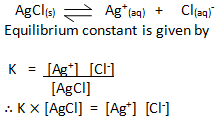Equilibrium Chemistry Class 11 Notes
Equilibrium Chemistry Class 11 Notes
These are Chemical Equilibrium revision notes of Equilibrium Chemistry Class 11 Notes. These notes provided for study purpose by ChemistryNotesInfo.com website.
CHEMICAL EQUILIBRIUM
Chemical reaction occur in one direction from reactant to products are termed as irreversible chemical reactions.
There direction is indicated by an arrow (→) pointing from reactant to products.
e.g.
- C(s) + O2(s) → CO2(S)
- AgNO3(aq) + NaCl(aq) → AgCl(S) ↓ + NaNO3(aq)
- 2KClO3(S) → 2KCl(S) + 3O2(S) ↑
Chemical reactions that do not go to completion in any direction and that can simultaneously occur in both forward (from reactant to product) and reverse (from product to reactant) direction are called reversible chemical reactions.
There direction is indicated by double arrows, one pointing in forward direction and other in reverse direction.
PHYSICAL EQUILIBRIUM
Physical equilibrium is defined as the equilibrium between two phase of the same substance when the rates of forward and reverse processes are equal.

The evaporation of liquid water in closed container is an example of physical equilibrium as the rate of evaporation and condensation are equal.
Another example of physical equilibriums are-
CHEMICAL EQUILIBRIUM
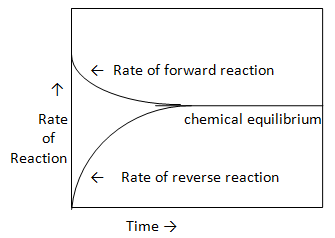
Chemical equilibrium is defined as the state of chemical reaction at which the rates of forward and reverse reactions are equal and the concentration of reactants and products reach constant values.
Consider a reversible reaction-
As reaction proceeds in forward direction with time, the concentration of A and B molecules decreases and concentration of C and D molecules increases. As the concentration of reactant molecules A and B decreases, the rate of forward reaction decreases and as the concentration of product molecules C and D increases the rate of reverse reaction increases.
After a certain time the rate of forward and reverse reaction gets equal and concentration of reactant and product reach constant values. At this state the chemical system is at equilibrium. As both forward and reverse reaction proceeds at the same rate therefore the chemical equilibrium is dynamic in nature.
LAW OF MASS ACTION
The law of mass action states that the rate chemical reaction is directly proportional to the product of concentrations (active mass) of the reactants, with each concentration is raised to a power equal to its stoichiometric coefficient in the balance equation.
Consider the general reaction
aA + bB → Products
According to the law of mass of action,
Rate α [A]a [B]b
Rate = K [A]a [B]b
The above equation represents rate equation and K is proportionality constant called rate constant.
EQUILIBRIUM CONSTANT (Kc)
The equilibrium constant (Kc) is defined as the ratio of the product of equilibrium concentrations (mol / L) of products to the product of equilibrium concentration of reactants, with the concentration of each substance raised to the power equal to its stoichiometric coefficient in the balanced chemical equation.
Consider the reversible chemical reaction

According to the law of mass action,
Rateforward α [A]a [B]b
Rateforward = Kf [A]a [B]b
Similarly Ratereverse α [C]c [D]d
Ratereverse = Kr [C]c [D]d
At equilibrium the rates of forward and reverse reaction are equal. Thus,
Rateforward = Ratereverse
Kf [A]a [B]b = Kr [C]c [D]d
- Kf / Kr = [C]c [D]d / [A]a [B]b = Kc
i.e Kc = Kf / Kr = [C]c [D]d / [A]a [B]b
The above equation represents equation for equilibrium constant Kc. Where Kf and Kr are the rate constant of forward and reverse reactions respectively.
You are learning Equilibrium Chemistry Class 11 Notes at ChemistryNotesInfo website – https://chemistrynotesinfo.com
Equilibrium constant with respect to partial pressure (Kp)
For reactions involving gases, the equilibrium constant is expressed in terms of partial pressure.
For the reaction
The equilibrium constant in terms of partial pressure (Kp) can be expressed by replacing the molar concentrations by partial pressures.
Kp = [PC]c [PD]d / [PA]a [PB]b
Where PA, PB, PC, PD are equilibrium partial pressures of A, B, C and D respectively.
Relationship between Kp and Kc
The partial pressure of each component in a mixture of ideal gaseous is directly proportional to its concentration at constant temperature.
For component A,
PAV = nART
PA = (nA/V) RT
Where (nA/V) = [A] is molar concentration in mol dm-3
PA = [A] RT
Similarly PB = [B] RT
PC = [C] RT
PD = [D] RT
Now substituting equation for PA, PB, PC, and PD in equation of equilibrium constant in terms of partial pressure we get,
Where Δn = (c+d) – (a+b) = number of moles of gaseous product – number of moles gaseous reactant
R = gas constant = 0.08206 L atm K-1 mol-1
Characteristics of equilibrium constant (Kc)
- The value of equilibrium constant (Kc) depends on the temperature.
- The value of equilibrium constant (Kc) does not depend upon original concentration of reactants or products, pressure, volume and catalyst.
- The values of equilibrium constant (Kc) are generally expressed without units.
- Higher values of Kc or Kp means more concentration of products is formed and the equilibrium point is more towards right hand side and vice versa.
Application of equilibrium constant
- To predict direction of reaction
If Qc < Kc, the reaction will proceed from left to right, in forward direction generating more product to attain the equilibrium.
If Qc = Kc, the reaction is at equilibrium and hence no net reaction occurs.
If Qc > Kc, the reaction will proceed from right to left, in reverse direction generating more reactant to attain the equilibrium.
- To know the extent of reaction
If Kc > 103, then the reaction is in favor of products and nearly goes to completion.
If Kc < 10-3, then the concentration of reactant is much greater than that of product. This shows that reaction does not take place.
If 103 > Kc > 10-3, then significant concentration of both reactants and products is present at equilibrium.
- To calculate equilibrium concentration
By knowing the value of equilibrium constant, the unknown concentration can be calculated.
Le – chatelier’s principal
It states that, when a system at equilibrium is subjected to a change in any of the factors determining the equilibrium conditions of a system, system will respond in such a way as to minimize the effect of change.
Factors affecting equilibrium
- Effect of change in concentration
If concentration of one or all of the reactant species is increased, the equilibrium shifts in the forward direction and more of the product are formed. Alternatively, if the product species is increased, the equilibrium shifts in the backward direction forming more reactant.
- Effect of temperature
Increase in temperature shifts the equilibrium in endothermic direction. And decrease in temperature shifts the equilibrium in exothermic direction.
- Effect of pressure
According to Le – chatelier’s principal, increase of pressure on a system at equilibrium shifts the equilibrium in the direction in which pressure reduced i.e. in direction where number of gaseous species is less.
- Effect of volume
Increase in pressure means decrease in volume, so the effect of change of volume is exactly reverse to that of pressure.
- Effect of catalyst
Catalyst has no effect on the equilibrium point because it speeds up both the forward and the backward reactions to the same extent.
IONIC EQUILIBRIUM
The equilibrium between ionic species in solution is called ionic equilibrium.
Water soluble compounds are classified as either electrolytes or non – electrolytes.
The electrolytes are compound that ionize (or dissociate) into their ions and conduct on electric current in aqueous solution.
The non – electrolytes are compound that do not ionize into their ions and hence do not conduct electric current in aqueous solution.
Strong and weak electrolytes
Electrolytes are classified as strong and weak electrolytes.
- Strong electrolytes
The substances which ionize almost completely into ions in aqueous solution are called strong electrolytes. For example HCl, H2SO4, HNO3, NaOH, KOH, NaCl, KNO3 etc. are strong electrolytes.
HCl + H2O → H3O+ + Cl–
HNO3 + H2O → H3O+ + NO3–
- Weak electrolytes
The substances which ionize to a small extent in aqueous solution are called weak electrolytes. For example, CH3COOH, NH4OH, HCN etc. are weak electrolytes.
In such cases, the molecules are in equilibrium with their ions.
Degree of dissociation
The degree of dissociation of an electrolyte is defined as the fraction of the total number of moles of the electrolytes that dissociates into its ion when an equilibrium is reached. It is given by expression,
α = Number of moles of electrolyte ionized as ion / Total number of moles of electrolytes
Percent dissociation = α x 100
Ostwald’s dilution law
If an electrolyte AB having concentration C moles per liter dissociates in aqueous solution and α is the degree of dissociation, then
The above mathematical relation is Ostwald’s dilution law.
- For weak acids
- Similarly, for weak base
ACIDS AND BASES
Arrhenius Theory
According to this theory,
Acid is a substance which contains hydrogen and produces H+ ions in aqueous solution.
For example : HCl(aq) → H+(aq) + Cl–(aq)
Base is a substance that contain OH group and produce OH– ions in aqueous solution.
For example : NaOH(aq) → Na+(aq) + OH–(aq)
This theory cannot be applied to compound which do not contains free H+ or OH– ions.
Bronsted – Lowery Theory
According to Bronsted – Lowery theory an Acid is any substance that can donate a proton (H+) to another substance and Base is any substance that can accept a proton (H+) from another substance. i.e. acid is proton donor and base is proton acceptor. For example
The pair of acids and bases which are formed from each other by the gain or loss of a proton are called conjugate acid – base pairs.
Lewis Theory
According to Lewis theory an acid is a substance which can accept a pair of electrons while a base is a substance which can donate a pair of electrons.
IONISATION OF WATER
K is the equilibrium constant for the ionization equilibrium.
Since the dissociation of water takes place to a small extent, the concentration of the undissociated water is nearly constant. Thus [H2O] = constant = K’
Therefore Kw = [H+] [OH–]
Where Kw is constant and is known as ionic product of water. At 298K, the value of
Kw is 1 x 10-14 mol2 L-2
Kw = [H+] [OH–]
= 1 x 10-14 at 298K
As the concentration of [H+] and [OH–] ions are equal in pure water,If [H+] = [OH–] : the solution is neutral
[H+] > [OH–] : the solution is acidic [H+] < [OH–] : the solution is basic
PH SCALE
The PH of a solution is defined as the negative logarithm, to the base I0, of the molar concentration of H+ ions in solution.
Mathematically, the pH of a solution is expressed as
PH = – log10[H+]
Hence [H+] = 10-pH
Similarly, poH of a solution is defined as the negative logarithm, to the base 10, of the molar concentration of OH– ions in solution.
Thus mathematically
POH = log10 [OH]
Relationship between PH and POH at 298K (25ᵒc)
PH + POH = 14
HYDROLYSIS
Hydrolysis is defined as reaction in which cations or anions or both ions of salt react with ions of water to produce acidity or alkalinity (basicity) or sometimes even neutrality.
- The aqueous solution of the salt of weak acid and strong base is alkaline.
- The aqueous solution of salt of weak base and strong acid is acidic.
- The aqueous solution of salt of strong acid and strong base is neutral.
- The aqueous solution of salt of weak base and weak acid may be acidic, basic or neutral depending upon relative strengths of acids and base.
BUFFER SOLUTION
Buffer solution is defined as a solution which resists change in pH on addition of small amounts of acids or bases or on dilution of the solution.
The ability of buffer solution to resist changes in pH value on addition of small amounts of either acid or base or on is called buffer action.
- A solution containing equimolar amounts of CH3COOH and CH3COONa is acidic buffer solution.
- A solution containing equimolar amounts of NH4OH and NH4Cl is basic buffer solution.
The pH of acidic and basic solution is given by Henderson-Hasselbalch equation for acidic buffer,
Buffer capacity is defined as the number of moles acid or base required to add one litre of solution to change the pH of unity.
Buffer capacity = Number of moles of acid or base added per liter / Change in pH
SOLUBILITY PRODUCT
When a saturated solution of a sparingly soluble salt is put into water, some of the salt dissolve in water and dissociates into its ion and most of the salt remains undissolved. Thus there is equilibrium between undissolved solid salt and its dissolved ions. This equilibrium is called solubility equilibrium.
For example AgCl, dissolves as-
As the concentration of [AgCl] is constant
COMMON ION EFFECT
The common ion effects state that the ionization of weak electrolyte is suppressed in presence of strong electrolyte containing an ion common to the weak electrolyte.
For example, in a solution containing weak base NH4OH and its soluble ion salt NH4Cl. In a weak base NH4OH there exist equilibrium and salt NH4Cl dissociates completely into its ion.
As the concentration of NH4+ ions increases then according to the Le Chatelier’s principal the equilibrium shifts towards left i.e. towards NH4 OH this produce non ionized NH4 OH molecule the result is that the ionization of NH4 OH is suppressed due to the presence of NH4CI containing a common NH4+ ion .
Write your experience about learning in comments below-