Amines Class 12 Notes
In this revision lecture notes, we will learn about Amines Class 12 Chemistry Notes. We will cover all basic scientific knowledge about Amines in this article. So, enjoy learning chemistry with Chemistry Notes Info @ https://chemistrynotesinfo.com
Amines Class 12 Chemistry Notes
AMINES INTRODUCTION
Amines are the organic derivatives of ammonia (NH3) in which one, two, or all three hydrogen atoms attached to nitrogen are replaced by equivalent number of same or different alkyl and/ or aryl groups.
Like ammonia, amines have pyramidal geometry and the nitrogen atom in amines, the nitrogen is attached to sp3 hybridized carbon of alkyl group and in aromatic amines to sp2 hybridized carbon of aryl group. H – N – H , C – N – H or C – N – C bond angle is less than 109ᵒ28’.
CLASSIFICATION OF AMINES
Depending upon the number of hydrogen atoms replaced by alkyl or aryl groups attached to nitrogen atom in ammonia molecule, amines are classified as –
1) Primary (1ᵒ) Amines
The functional group present is – NH2 (Amino group)
e.g. CH3 – NH2 Methylamine
2) Secondary (2ᵒ) Amines
The functional group present is – NH- (Amino group)
e.g. CH3 – NH– CH3 Dimethylamine
3) Tertiary (3ᵒ) Amines
The functional group present is -N= (Tertiary nitrogen atom)
Secondary and tertiary amines are further classified as
Secondary and Tertiary amines of Amines Class 12 Notes are further classified into two groups i.e.
a) Simple / Symmetrical amines
In simple amines same alkyl or aryl groups are attached to the nitrogen.
b) Mixed / Unsymmetrical amines
In mixed amines different alkyl or aryl groups are attached to the nitrogen.
METHOD OF PREPARATION OF AMINES
1) By ammonolysis of alkyl halides
When ethyl bromide is heated with alcoholic ammonia at 373K in sealed copper tube, it gives mixture of ethylamine, diethyl amine and triethyl amine along with tetraethyl ammonium bromide.
C2H5 – Br + NH3 Δ → C2H5 – NH2 + HBr
C2H5 – NH2 + C2H5 – Br Δ → (C2H5)2NH + HBr
(C2H5)2NH + C2H5 – Br Δ → (C2H5)3N + HBr
(C2H5)3 + C2H5 -Br Δ → (C2H5)4N+Br–
Thus,
NH3 RX→ R – NH2 RX→ R2NH RX→ R3N RX→ R4N+X–
2) By reduction of Nitro Compounds
Both aliphatic and aromatic primary amines can be prepared by the reduction of nitro compounds by either catalytically with H2 in the presence of Raney Ni, Pt or Pd or chemically with active metals.
e.g. CH3NO2 + 3H2 Raney Ni/Pt→ CH3NH2 + 2H2O Nitromethane Ethanol Methylamine
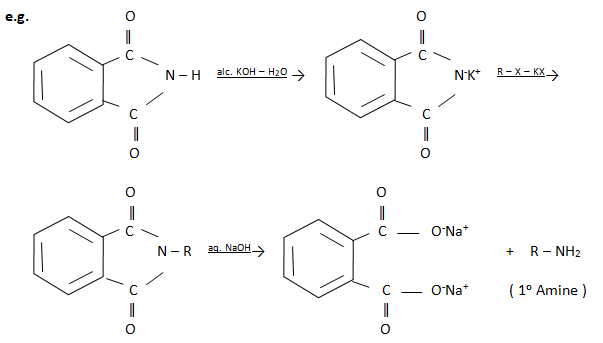
3) By Gabriel phthalimide synthesis
Phthalmide reacts with ethanolic potassium hydroxide to give potassium salt of phthalmide. In this step N – H proton is removed to give imide ion. It is then heated to give N – alkyl phthalimide, which on alkaline hydrolysis give a primary amine. Share these Amines Class 12 Notes of chemistry with your friends.
4) By Reduction of Alkyl nitrites / cyanides
Nitriles on reduction with lithium aluminium hydride (LiAlH4) or catalytic hydrogenation produce primary amines.
R – CN (Alkyl cyanide) — H2 / Ni & Na(Hg) / C2H5OH → R – CH2 – NH2 (1ᵒ Amine)
5) By reduction of amides
Acid amides on reduction with lithium aluminium hydride give corresponding amines.
6) By Hoffmann bromamide degradation
The conversion of amides into amines in the presence of bromine and alkali is known as Hoffmann degradation of amides.
R – CO – NH2 (Amides) + Br2 + 4NaOH → R – NH2 (Amine) + Na2CO3 + 2NaBr + 2H2O
PHYSICAL PROPERTIES OF AMINES
1) Aliphatic amines with low molecular weight are colourless, gaseous compounds with fishy odour. High molecular weight aliphatic amines are solid.
2) Pure aromatic amines such as aniline are colourless liquid. Arylamines are toxic in nature.
3) Aliphatic amines are soluble in water. As the molar mass increase solubility decreases.
4) Amines are less polar than the corresponding alcohol but more polar than corresponding alkanes.
5) Amines are higher boiling points than corresponding alkanes but lower than corresponding alcohols or carboxylic acids. The order or boiling points of isomeric amines is 1ᵒ > 2ᵒ > 3ᵒ.
REACTIONS OF AMINES
The lone pair of electron on nitrogen makes amines both basic and nucleophilic. They react with acids to form salts and react with nucleophiles in many reactions.
1) Basic nature of Amines
Nitrogen atom of amines contains a lone pair of electron which can be donated. Thus, amines act as Lewis bases and are Lowery – Bronsted bases as they accept a proton.
The parent amine is regenerated when alkyl ammonium halide is treated with NaOH.
R – NH3+X– (Alkyl Ammonium Halide) + NaOH → R – NH2 (Amine) + NaX + H2O
Amines are weak bases. They dissolve in water and produce OH– ions. Aqueous Solutions of amines turn the color of litmus paper from red to blue.
R – NH2 + H – O – H ↔ R – NH3+ + OH–
Weak Base Strong base
The equilibrium lies far to the left as OH– is a stronger base than amines. The expressions for the equilibrium constant K, basicity constant Kb and pKb values are as follows.

PKb = -logKb
Strong bases have high values of Kb and low values of PKb.
2) Action of nitrous acid
- On primary (1ᵒ) amines
Except methylamine, primary amines react with nitrous acid, in cold condition to give alcohol and nitrogen gas.
R – NH2 (1ᵒ amines) + NaNO2 + 2HCl 273 – 278K→ R – OH + HCl + N2 ↑
- On secondary (2ᵒ) amines
Secondary amines react with nitrous acid to give the N – nitrosoamines which are generally pale yellow oils.
R2 – NH (2ᵒ amine) + HNO2 —NaNO2 + dil. HCl (at 273 – 278K)
→ R2N – N = O + H2O
- On tertiary (3ᵒ) amines
Tertiary amines react with nitrous acid to form water soluble nitrite salt. As visible change observed, it is said that there is no reaction.
R3N (3ᵒ amine) + HNO2 NaNO2 / dil. HCl ( at 273 – 278K )
→ [ R3NH ]+NO–2 (No change)
3) Acylation of amines
Acylation is the replacement of a hydrogen atom of amino group by acyl group (R – CO). It is nucleophilic substitution reaction. Acetylation of primary / secondary amines with acetyl chloride or acetic anhydride gives corresponding amine.
4) Carbylamine reaction
Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines. This reaction is known as carbylamines reaction or isocyanides test.
R – NH2 + CHCl3 + 3KOH Heat→ R – NC + 3KCl + 3H2O
5) Reaction with arylsulphonyl Chloride (Hinsberg’s test)
- Primary amines when reacts with benzene Sulphonyl chloride ( Hinsberg’s reagent),
it yields N – ethylbenzene sulphonamide.
- Secondary amines reacts with benzene sulphonyl chloride, it yields N, N – dimethylbenzene sulphonamides.
- Tertiary amines do not react with benzene sulphonyl chloride.
6) Electrophilic aromatic substitution
- Bromination
Reaction of aniline with bromine water at room temperature results in the formation of white precipitate of 2, 4, 6 – tribromoaniline.
- Nitration
In direct nitration of aniline with concentrated nitric acid at 288K in the presence of sulphuric acid, a mixture of ortho, meta and para isomers of nitroaniline are obtained as product. Also, dark coloured tars are obtained due to oxidation.
- Sulphonation
Reaction of aniline with cold concentrated Sulphuric acid gives anilinium hydrogen sulphate. Heating anilinium hydrogen sulphate with Sulphuric acid at 453K to 473K gives p – aminobenzene sulfonic acid (sulphonic acid) as major product.
DIAZONIUM SALTS
The diazonium salts have the general formula RN2+X– where R stands for aryl group and X– ion may be Cl–, Br–, HSO4, BF4–, etc.
The N2+ group is called diazonium group.
e.g. C6H5N2+Cl– IS named as benzenediazonium chloride.
The conversion of primary aliphatic or aromatic amines into diazonium salts is known as diazotization.
PREPARATION OF DIAZONIUM SALTS
Diazonium salts are prepared by the reaction of nitrous acid in cold condition with alkyl/aryl primary amines (other than methylamine).
e.g.
REACTION OF DIAZONIUM SALTS
Reaction involving displacement of nitrogen (Diazonium group)
- Replacement by -Cl , -Br and –CN
The reaction in which copper (1) salts are used to replace nitrogen in diazonium salt is called sandmeyer reaction
Yield in sandmeyer reaction is better than yield in Gattermann reaction.
- Replacement by – I
Ar – N+2X– + KI Δ → Ar – I + KX + N2 ↑
- Replacement by – F
Ar – N+2X– HBr → Ar – N+2BF–4 Δ → Ar – F + BF3 + N2 ↑
- Replacement by – H
Ar – N+2X + H3PO2 + H2O CuCl → ArH + H3PO3 + HX + N2 ↑
- Replacement by – OH
Ar – N+2X + H2O Dil. H2SO4 → Ar – OH + HX + N2 ↑
- Replacement by – NO2
Ar – N+2X HBF4 → Ar – N+2BF–4 NaNO2 / Cu & Δ → Ar – NO2 + NaBF4 + N2 ↑
IMPORTANCE OF DIAZONIUM SALTS
1) Arene diazonium salts can be prepared from nearly all primary amines and are helpful in the synthesis of variety of organic compounds.
2) Arenes diazonium salts are used as useful intermediates to introduce various groups into aromatic ring. Such as – F, – Cl, -Br, – I, -CN, -NO2, -OH, -H, etc.
3) The halide or Cyano group can be easily introduced in aromatic ring through diazonium salts.
4) The compounds which cannot be prepared by direct electrophilic aromatic substitution can be prepared by replacement of diazo group.
5) Azo compounds obtained from diazonium salts are strongly coloured and are used as dyes.
Like Subscribe and Share these Amines Class 12 Notes by Chemistry Notes Info with your friends…














%20(1).png)
