Hydrogen Class 11th Chemistry Notes
Hydrogen is the first element in the periodic table and is the lightest element. It exists as a diatomic molecule (H2) and is called dihydrogen. It has one proton and one electron, and it has electronic configuration 1s1. This electronic configuration is responsible for dual nature of hydrogen i.e. hydrogen shows resemblance with alkali metals as well as halogens.
Resemblance of hydrogen with alkali metals
Like alkali metals –
·
Hydrogen exhibits electropositive character i.e. it has
tendency to lose electron.
·
The normal valency of hydrogen is 1.
·
Hydrogen forms halides, oxides and sulphides.
·
Hydrogen acts as strong reducing agent.
Resemblance of hydrogen with halogens
Like halogens-
- Hydrogen exhibits electronegative character by accepting one electron to complete its valance shell.
- Hydrogen shows same order of ionisation energy (IE1) as that of halogens.
- Hydrogen (H2) is a gas in molecular state similar to F2 and Cl2.
- Hydrogen shows -1 oxidation state in its compounds with more electropositive element.
POSITION OF HYDROGEN IN THE PERIODIC TABLE
As
the hydrogen resembles both the alkali metals and the halogens and also differs
from them. Also hydrogen exhibits some unique behavior therefore the position
of hydrogen is uncertain and it is placed separately in periodic to table.
ISOTOPES OF HYDROGEN
Hydrogen
has three isotopes, protium (11H), deuterium (12H)
and tritium (13H).
i.
Protium or ordinary hydrogen (11H).
It is the most abundant isotope of
hydrogen (99.985%). Its nucleus has one proton and no neutron (mass no. = 1).
ii.
Deuterium or heavy
hydrogen (12H or D).
It is less abundant and is present in heavy water (D2O). Its
nucleus has one proton and one neutron (mass no. = 2).
iii.
Tritium (13H or T)
It is the rarest isotope of hydrogen and is radioactive in nature. Its
nucleus has one proton and two neutrons (mass no. = 3).
PREPARATION OF DIHYDROGEN (H2)
Laboratory methods
1. Action of dilute HCl or H2SO4
on granulated zinc.
Zn +
2HCl → ZnCl2 +
H2 ↑
2. Action of dilute H2SO4
on magnesium ribbon.
Mg +
H2SO4 → MgSO4 +
H2 ↑
3. Action of water on sodium
hydride.
NaH +
H2O → NaOH
+ H2 ↑
4. By action of KOH of scrap aluminum
or silicon. (Uyeno’s method)
2Al
+ 2KOH +
2H2O → 2KAlO2 + 3H2 ↑
Commercial method
1) From water gas (Bosch process)
Water gas (CO + H2) is
mixed with the steam and the gaseous mixture is passed over heated catalytic
mixture of ferric oxide (Fe2O3) and chromium oxide (Cr2O3)
at 773K.
H2 +
CO + H2O Fe2O3 + Cr2O3 (773K) →
CO2 + 2H2
Water gas Steam
2) From Steam (Lanes process)
When
superheated steam is passed over iron filling heated to 1023 – 1073K hydrogen
is formed.
3Fe + 4H2O(steam) (1023 – 1073)K (Fe)→ Fe2O4 + 4H2(g)
3) Hydrocarbon steam process
Hydrogen
is prepared by the action of steam of hydrocarbon at 1270K.
CH4 + H2O 1270K → CO
+ 3H2
4) By electrolysis of water
Electrolysis
of acidified water using platinum electrodes gives dihydrogen. Here dihydrogen
is librated at the cathode while dioxygen is librated at anode.
CHEMICAL PROPERTIES OF DIHYDROGEN
1)
Dihydrogen reacts with halogens (X2) to give hydrogen halide (HX).
H2 + X2 →
2HX ( X = F, Cl, Br, I )
2)
Dihydrogen reacts with dioxygen to forms water. This reactions is highly
exothermic.
2H2 + O2 catalyst or heating →
2H2O . ΔH = -285.9 KJ.mol-1
3)
With dinitrogen dihydrogen forms ammonia. This process is coiled Hober process
of
manufacture of ammonia.
3H2 + N2 673K, Fe catalyst, 200atm →
2NH3
4)
Dihydrogen combines with many metals at high temperature to yield the
corresponding
hydrides
2Na
+ H2 →
2NaH (sodium hydride)
5)
It acts as reducing agent and thus reduces certain oxides of metals.
ZnO
+ H2 → Zn + H2O
Fe2O4 + 4H2 →
3Fe + 4H2O
6)
Hydrogenation of vegetable oils using nickel as catalyst gives edible facts
(margarine and Vanaspati
ghee)
Vegetable oil + H2 Finely divided Ni, 450K, 8-10atm →
Solid fat
USES OF DIHYDROGEN
·
Dihydrogen is used in the preparation of ammonia by Haber’s
process.
·
It is used in the hydrogenation of vegetable oils.
·
It is used for the manufacture of metal hydrides.
·
It is used as rocket fuel in space research.
·
Dihydrogen is used in fuel cell for generating electrical
energy.
·
It is used in the atomic hydrogen torch and oxy hydrogen
torches for cutting and welding.
HYDRIDES
The
binary compounds of hydrogen with other elements are called hydrides. These
hydrides have the formula EHx or EmHn (E =
Element). These are classified into three types.
a)
Ionic or saline hydrides
b)
Covalent or molecular hydrides
c)
Metallic or nonstoichiometric or interstitial hydrides
a) Ionic or saline hydrides
These
are formed by the combination of hydrogen with metals which have low electro
negativity values and are electropositive with respect to hydrogen. These
includes elements of S block element. Ionic hydrides are prepared by the direct
combination of metals.
e.g.
LiH, KH, CaH2, MgH2 etc.
b) Covalent or molecular hydrides
These
hydrides are formed by the combination of elements of comparatively higher
electro –negativity as p-block element. The bonds formed are mostly covalent in
character. Covalent hydrides are generally volatile.
e.g.
H2O, CH4, NH3, HF etc.
c) Metallic or nonstoichiometric or interstitial hydrides
Metallic
hydrides are formed by many d - block and f - block elements. Elements of group 7, 8, 9 of d –
block do not form hydrides and this is referred as hydride gap. Metallic
hydrides are non – stoichiometric and show electric conductance. In these
hydrides, hydrogen occupies interstices in the metal lattice producing
distortion without any change in its type. Therefore, they are termed as
interstitial hydrides.
e.g.
SCH2, TiH2, VH, ZrH2
WATER (H2O)
Water
is essential to all forms of life. It is most common, abundant and easily
obtainable of all chemical compounds. It is regarded as universal solvent.
Water (H2O) is hydride of oxygen. In nature, water exist in three
physical states, water (liquid), Ice (Solid) and water vapour (gas).
Structure of water
Water
molecule is a bent molecule with bond angle of 104.5ᵒ and O-H bond length is
95.7pm.
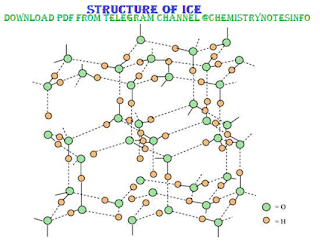
Structure of Ice
Ice
is the solid form of water. It has a highly ordered three dimensional hydrogen
bonded structure. In ice each oxygen atom is surrounded tetrahedrally by four
other hydrogen atoms at a distance of 276pm. The strength of hydrogen bonding
in ice is more than liquid water. There is empty space in crystal of ice due to
hydrogen bonding. This makes density of ice lower than liquid water, hence ice
floats on water.
Chemical properties of water
1) Dissociation of water
Water
dissociates into its ion.
H2O + H2O →
H3O+
+ OH-
Hydronium ion
2) Amphoteric nature
Water
has the ability to act as on acid as well as base. Such kind of behavior is
known as amphoteric nature.
H2O +
NH3 → OH- + NH4+
H2O +
HCl → H3O+ +
Cl-
3) Oxidizing and reducing nature
Water
can act both as an oxidizing and reducing agent in its chemical reactions. With
active metals water behaves as an oxidizing agent while with highly
electronegative element, it acts as a reducing agent.
2Na + 2H2O →
2NaOH + H2
Oxidizing agent
2F2 +
2H2O → 4HF
+ O2
Reducing agent
4) Formation of Hydrates
Water
has strong ability to form compounds with some metal salts known as hydrates. This
hydrates can be classified into three types:
i) Coordinated water
[Cr(H2O)6]3+3Cl-
ii) Interstitial water
BaCl2.2H2O
iii) Hydrogen – bonded water
[Cu(H2O)4]2+SO42-.H2O in
CuSO4.5H2O
HEAVY WATER
Heavy
water is deuterium oxide (D2O). It was discovered by Urey in 1932.
The reaction of D2O are slightly slower than H2O. Ordinary
water contains one part of heavy water in 6000 part of it. It is used as a
moderator in nuclear reactors. It is used as tracer compound in determining the
mechanism of many organic reactions.
HARD AND SOFT WATER
Natural
water contains dissolved salts. Depending upon its behavior towards soap
solution, water may be classified as hard water and soft water.
a) Soft water :
Water which produces lather with soap solution readily is
called soft water. For example : distilled water, rain water, and
demineralized water.
b) Hard water :
Water which does not produces lather with soap solution
readily is called hard water. For example : sea water, river water, well water
and tap water.
The
hardness of water is due to the presence of the bicarbonates, chlorides and
sulphates of calcium and magnesium.
Isotopic varieties of water
Ordinary
water contains 18 different kinds of water. Such as variety is possible due to
the different isotopic forms of hydrogen and oxygen. Hydrogen has three
isotopes H(protium), D(Deutrium), T(tritium) and oxygen also has three isotopes
16O, 17O, 18O. These isotopes of hydrogen and
oxygen combine to give 18 different kinds of water from which H2O is
most abundant.
HYDROGEN PROXIDE (H2O2)
Hydrogen
peroxide was discovered by Thenard in 1818. It is an important chemical used in
pollution control treatment of domestic and industrial effluents.
Preparation of H2O2
1)
In laboratory H2O2 is prepared by acidifying barium
peroxide and removing excess water by evaporation under reduced pressure.
BaO2.8H2O + H2SO4 →
BaSO4 + H2O2 +
8H2O
2)
By the action of dilute acid on sodium peroxide (Merck’s method)
Na2O2 + H2SO4 →
NaSO4 + H2O2
3)
By bubbling CO2 through a paste of BaO2
BaO2 + H2O + CO2 →
BaCO3 ↓ + H2O2
4)
By the action of phosphoric acid on BaO2
3BaO2 +
2H3PO4
→ Ba3(PO4)2
↓ +
3H2O2
5)
Industrially H2O2 is prepared by the auto – oxidation of
2 – Ethyl anthraquinol.
2-ethyl anthraquinol O2 (air) (H2/Pd) → 2-ethyl anthraquinol + H2O2
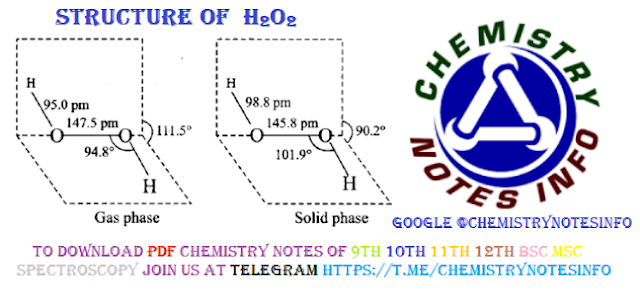
Structure of H2O2
It is non–planar open book (Skew) structure. The bond length and bond angle are slightly different in gas and solid phase due to hydrogen bonding. Structure of H2O2 HYDROGEN PROXIDE.
Chemical Properties of H2O2
1)
It decomposes rapidly on heating in presence of finely divided metals, such as
Ca, Fe, Cu, Au, Ag, Pt, MnO2, Carbon, dust, light, etc.
H2O2 + H2O2 →
2H2O + O2 (ΔH = -196KJ)
2)
It acts as an oxidizing as well as reducing agent in both acidic and alkaline or
basic medium. Some example are given below
i)
Oxidizing action in acidic medium
PbS + 4H2O2 →
PbSO4 + 4H2O
ii)
Reducing action in acidic medium
HOCl + H2O2 →
H3O+
+ Cl- + O2
iii)
Oxidizing action in alkaline medium
Mn2+ + H2O2 →
2Fe3+ + 2OH-
iv)
Reducing action in alkaline medium
Cl2 + H2O2 + 2OH- → 2Cl- +
2H2O + O2
3)
Hydrogen peroxide acts as a bleaching agents due to the release of nascent
oxygen.
H2O2 →
H2O + O
4)
Hydrogen peroxide undergoes addition reaction with alkenes to form glycols.
Uses of H2O2
·
It is widely used in environmental chemistry.
·
It is used as a antiseptic and is sold in the market as
perhydrol.
·
It is used in daily life as a hair bleach and as mild
disinfectant.
·
It is used as a bleaching agent and to manufacture chemicals
like sodium perborate and percarbonate.




%20(1).png)
