Hydrocarbon Class 11th Chemistry Notes
HYDROCARBONS
The compounds which contain only carbon and hydrogen are called hydrocarbons. Hydrocarbons are classified as aliphatic, alicyclic and aromatic hydrocarbons.
- Aliphatic hydrocarbons contain open chains of carbon atoms, which are further classified as saturated and unsaturated hydrocarbons.
- Saturated hydrocarbon contains all C – C single bonds (alkanes) while unsaturated hydrocarbons contain C = C double bonds (alkenes) or C ≡ C triple bonds (alkynes).
- Alicyclic hydrocarbons contain rings of carbon atoms and have properties similar to that of aliphatic hydrocarbons.
- Aromatic hydrocarbons contain one or more benzene rings in their structure.
ALKANES
Alkanes are aliphatic saturated hydrocarbons containing carbon – carbon and carbon – hydrogen single covalent bonds. Alkanes are also called paraffins because alkanes do not undergo chemical reactions easily.
The general chemical formula of alkanes is CnH2n+2, where n is an integer and indicates the number of carbon atoms in the molecule. Some common alkanes are methane (CH4), ethane (C2H6), propane (C3H8), Butane (C4H10), Pentane (C5H12) etc. Every carbon atom in alkanes is sp3 hybridized and has tetrahedral geometry.
The tetrahedral are joined together and C – C and C – H bond lengths are 154pm and 112 pm respectively. The H – C – H, C – C – H and C – C – C bond angles are 109ᵒ28’.
CONFORMATION IN ALKANES
The different spatial arrangements of atoms in a molecule that can be converted into one another by free rotation about single bonds are called conformations or conformers or rotamers. These isomers cannot be usually isolated because they interconvert rapidly. Conformations can be represented by different formula, such as sawhorse and Newmann projection formula.
METHODS OF PREPARATION OF ALKANES
From unsaturated hydrocarbons : By hydrogenation
Alkanes are obtained by passing a mixture of alkenes or alkynes and hydrogen over Raney nickel and catalyst at about 473K to 573K. This reaction is called Sabatier and Sanderson reaction. Platinum or Palladium also catalyses the reaction at room temperature.
e.g. CH2 = CH2 + H2 Raney Ni & Δ → CH3 – CH3
Ethene Ethane
CH ≡ CH + 2H2 Raney Ni & Δ → CH3 – CH3
Ethene Ethane
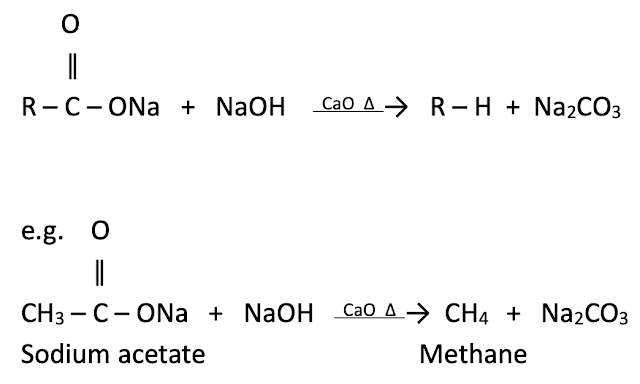
From sodium of fatty acids : By decarboxylation
The reaction in which carbon dioxide is removed from carboxylic acid is called decarboxylation. When anhydrous sodium salt of fatty acid is fused with soda lime it gives alkane containing one carbon atom less than carboxylic acid.
By reduction of alkyl halides
Alkyl halides on reduction with zinc and dilute hydrochloric acid from alkane. The reduction of alkyl halides is due to nascent hydrogen obtained from reducing agent. Zn – Cu couple and alcohol can also be used as the reducing agents.
R – X + 2[H] zn, HCl → R – H + HX
Alkyl halide Alkane
e.g. CH3 – I + 2[H] Zn, HCl → CH4 + HI
Methyl iodide Methane
By using active metal (Wurtz synthesis reaction)
Alkyl halides on heating with sodium metal in presence of dry ether as solvent give higher alkanes containing even number of carbon atoms.
R – X + 2Na + X – R dry ether → R – R + 2NaX
e.g. CH3 – Cl + 2Na + Cl – CH3 dry ether → CH3 – CH3 + 2NaCl
Methyl Chloride Ethane
PHYSICAL PROPERTIES OF ALKANES
- Alkanes are colourless and odourless they are insoluble in water, but readily dissolve in non – polar solvents like benzene, ether, chloroform etc.
- At room temperature, the first four alkanes are gases, next thirteen (C5 to C17) are liquids where as higher alkanes are waxy solids.
- Alkanes are non polar as C – H and C-C bonds in alkanes are nonpolar covalent bonds. All alkanes are less dense than water and their relative density increases with increase in molecular mass.
- Melting and boiling points increases with increase in molecular mass and decreases as branching increases.
CHEMICAL PROPERTIES OF ALKANES
Halogenations of alkanes
Alkanes reacts with halogens in presence of ultraviolet light as diffused sunlight or at high temperature (573K to 773K) to give mixture of alkyl halides.
Chlorination of Methane :
CH4 Cl2 – HCl → CH3 – Cl Cl2 – HCl → CH2Cl2 Cl2 – HCl → CHCl3 Cl2 – HCl → CCl4
Nitration of alkanes
The reaction in which one hydrogen atom of alkanes in replaced by nitro (- NO2) group is called nitration of alkanes. It is carried out in vapour phase, by heating a mixture of an alkane and conc. HNO3 at about 423K to 698K.
e.g. CH3 – CH3 + HO – NO2 423 to 698K → CH3 – CH2 – NO2 + H2O
Ethane Nitroethane
Combustion of alkanes
Alkanes on heating in the presence of air or dioxygen (O2) are completely oxidized to CO2 and water with the evolution of large amount of heat.
e.g. CH4 + 2O2 → CO2 + 2H2O
Methane
ΔcHᵒ = -890KJmol-1
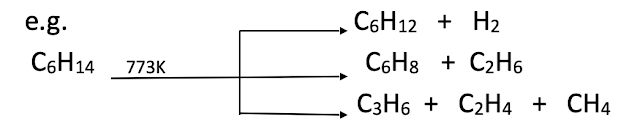
Pyrolysis of alkanes
Alkanes on heating at higher temperature in absence of air decompose to give lower alkanes, alkenes and hydrogen etc. this is known as pyrolysis or cracking.
Reforming of alkanes
Alkanes having more than five carbon atoms get cyclized to benzene and its homologues, on heating under 10 to 20 atm. pressure at about 773K in presence of oxides of Cr, V, Mo supported on alumina. This reaction is called aromatization or reforming.
ALKENES
Alkenes are aliphatic unsaturated hydrocarbons containing one or more double bonds. The aliphatic unsaturated hydrocarbons are called alkadienes and alkatrienes respectively. Alkenes are also called Olefines (oil forming). The general formula of alkenes is CnH2n.
Every doubly bonded carbon atom in alkene is sp2 hybridized and has trigonal planar geometry with bond angles 120ᵒ. In alkenes the doubly bonded carbon atoms have one C – C sigma (σ) and one C – C pi (∏) bond. The ∏ bond enthalpy is 284 KJmol-1 and that of (σ) bond is 397KJmol. The C = C bond length in ethane is 133 pm.
METHOD OF PREPARATION OF ALKENES
From alcohols (by dehydration)
Dehydration by concentrated sulphuric acid
When an alcohol is heated with concentrated sulphuric acid (H2SO4) at about 443K, it loses a water molecule to give corresponding alkene. It is acidic dehydration.
Dehydration by alumina
When vapours of an alcohol are passed over heated alumina at 623K, it gives corresponding alkenes. It is known as vapour phase dehydration of alcohol.
From alkyl halides (By dehydrohalogenation)
When an alkyl halide is boiled with alcoholic alkali it gives corresponding alkene. The order of reactivity of alkyl halides towards dehydrohalogenation is 3ᵒ > 2ᵒ > 1ᵒ and that of halogens is I > Br > Cl.
e.g. CH3-CH2-Br + KOH boil ---> CH2=CH2 + KBr + H2O
Ethyle bromide Ethyne
From vicinal dihalides (By dehalogenation)
The dihalides in which two halogen atoms are attached to the adjacent carbon atoms are called vicinal dihalides when vicinal dihalide is heated with zinc metal in presence of alcohol, it gives alkene.
e.g. Br-CH2-CH2-Br + Zn Alcohol ---> CH2=CH2 + ZnBr2
Ethylene dibromide Ethene
From alkynes
Alkynes on partial reduction by Lindlar’s catalyst gives alkenes. Lindlar’s catalyst is metallic palladium deposited on calcium carbonate, partially poisoned with lead acetate.
e.g. CH=CH + H2 Pd, Lead acetate ---> CH2=CH2
Ethene Ethene
PHYSICAL PROPERTIES OF ALKENES
- Alkenes resemble alkanes in most of their physical properties. Ethyne is colourless gas with sickly sweet odour while all other alkenes colourless and odourless.
- First three alkenes are gases, next 14 are liquids and rest higher ones are solids. Alkenes are lighter than water and the density gradually increases with rise in molecules mass.
- Alkenes are non – polar or weakly polar compounds and are insoluble in water but soluble in non – polar solvents like benzene, ether, chloroform, etc.
- As in alkanes, boiling point of alkenes increases with increase in molecular mass and decreases with increase in branching.
CHEMICAL PROPERTIES OF ALKENES
Formation of alkanes (Hydrogenation)
Alkenes on catalytic hydrogenation at about 443K to 453K, under pressure give corresponding alkanes.
e.g. CH2=CH2 + H2 Raney Ni, Δ ----> CH3-CH3
Ethene Ethane
Formation of vicinal dihalides (Halogenation)
Alkenes react with halogens to give vicinal dihalide. The order of reactivity of halogens is Cl2 > Br2 > I2.
e.g. . CH2=CH2 + Cl2 CCl4 ---> Cl- CH2-CH2-Cl
Ethene Ethylene dichloride
Formation of alkyl halides (Hydrohalogenation)
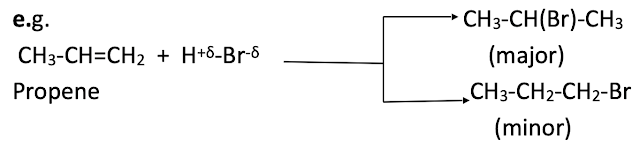
Alkenes react with hydrogen halides to give corresponding alkyl halides. In case of symmetrical alkenes, addition of hydrogen halides leads to only one product. However unsymmetrical alkenes give two products. The major product is predicted by following rule.
Markownikoff’s rule:
When an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of reagents gets attached to that carbon atom which carries less number of hydrogen atoms.
Peroxide effect Or anti-Marjownijoff's rule:
If addition takes place in presence of peroxide then negative part of reagent gets attached to that carbon atom which carries greater number of hydrogen atoms.
Addition of sulphuric acid
Alkenes react with cold conc. Sulphuric acid to give alkyl hydrogen sulphate which on boiling with water give corresponding alcohol. Addition of alkyl hydrogen sulphate takes place according to morkownikoff’s rule.
CH2=CH2 (Ethene) + H-OSO3H (Conc.) ----> CH3-CH2-OSO3H (Ethyl hydrogen sulphate) + H-OH boil---> CH3-CH2-OH (Ethyl alcohol) + H2SO4
Formation of aldehydes and ketones (Ozonolysis)
When a stream of ozonized oxygen is passed through a solution of alkenes in presence of organic solvent, it gives unstable ozonide which on reduction with zinc dust and water give either aldehyde or ketones or mixture of both.
Formation of glycols : Hydroxylation (Bayer’s test)
Alkenes react with cold and dilute alkaline potassium permanganate to give vicinal glycols (1, 2 - diols).
e.g. CH2=CH2 + H2O + (O) Alkaline KMnO4---> HO-CH2-CH2-OH
ethane Ethylene glycol
Polymerization
Ethene on polymerization at high temperature and pressure in presence of oxygen gives high molecular weight polymer called polythene.
e.g. CH2=CH2 ---Polymerization----> –[--CH2-CH2--]-
ethane Polythene
ALKYNES
Alkynes are aliphatic unsaturated hydrocarbons containing at least one triple bond. The aliphatic unsaturated hydrocarbons containing two or three carbon – carbon triple bonds in their structure are called alkadiyenes and alkatriynes respectively. The general formula of alkynes is CnH2n-2 carbon atom in ethylene is sp hybridized and has linear geometry.
Acetylene also called as ethyne is the chemical compound with the chemical formula C2H2 and have chemical structure H−C≡C−H. Ethyne is a hydrocarbon and it is the simplest
The H – C – C bond angle is 180ᵒ and C≡C (823KJmol-1) bond length is 120pm. The triply bonded (C≡C) carbon atoms have one C – C sigma (σ) bond and 2 C – C pi (∏) bonds.
METHOD OF PREPARATION OF ALKYNES
From calcium carbide (Industrial method)
Industrially the ethyne is prepared by the reaction of calcium carbide with water.
CaC2 + 2H2O ----> C2H2 + Ca(OH)2
By dehydrohalogenation
From vicinal dihalides
When vicinal dihalides react with alcoholic solution of potassium hydroxide to form vinyl halide which on further treating with sod amide forms alkyne.
e.g.
Br-CH2-CH2-Br (Ethylene dibromide) + KOH ----> CH2=CH-Br NaNH2 ---> CH≡CH (ethyne) + NaBr +NH3
From germinal dihalide
When germinal dihalide is boiled with alcoholic caustic potash it gives vinyl halide which on heating with sod amide gives acetylene.
e.g.
CH-CH(Br)2 + KOH Δ ---> CH2=CH-Br NaNH2---> CH≡CH + NaBr +NH3
From tetra halide (by dehalogenation)
When acetylene tetra bromide is heated with zinc is presence of alcohol, acetylene is formed.
(Br)2-CH-CH-(Br)2 + 2Zn Alcohol---> CH≡CH (Ethyne) + ZnBr2
PHYSICAL PROPERTIES OF ALKYNES
- Alkynes resemble alkanes and alkenes in their physical properties. First three alkynes are gases, next eight are liquids and higher ones are solids.
- All alkynes, except acetylene, are odourless. They are generally more polar and have slightly higher boiling points than corresponding alkanes and alkenes .
- They are less dense than water and are insoluble in water but quite soluble in organic solvents like ether, benzene, etc.
CHEMICAL PROPERTIES OF ALKYNES
Acidic character of alkynes
Alkynes react alkali metal sodium to give sodium acetylide with the liberation of hydrogen gas while alkanes as well as alkenes do not react with alkali metals. This reaction explains acidic character of alkynes.
CH≡CH + 2Na ----> 2 CH≡C-Na+ + N2
Formation of alkanes (Hydrogenation)
Alkynes on hydrogenation in presence of catalyst like raney Ni to give first alkene which further reaction give alkanes.
CH≡CH (Ethyne) + H2 Raney nickel Δ---> CH2=CH2 raney nickel H2---> CH3-CH3(Ethane)
Formation of tetra halides (halogenations)
Ethyne on halogenations first gives ethyne dichloride which on further reaction gives ethyne tetrachloride.
CH≡CH (Ethyne) + Cl2 ----> (Cl)2-CH-CH-(Cl)2
Formation of aldehydes or ketones (hydration)
Alkynes react with water in presence of 40% sulphuric acid and 1% mercuric sulphate to form aldehyde or ketones i.e. carbonyl compounds.
CH≡CH (Ethyne) + H-OH ----> CH3-CHO (Acetaldehyde)
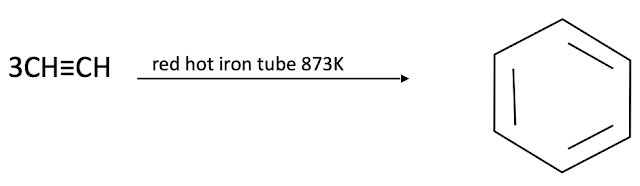
Formation of benzene (cyclization)
When ethyne is passed through red hot iron quartz tube at873K, it trimerises to give benzene.
AROMATIC COMPOUNDS
Benzene and compounds that resembles benzene in their chemical properties are called aromatic compounds or arenes. Aromatic compounds posses pleasant fragrance (Aroma). Benzene is the parent compound in most of the aromatic compounds. Benzene was originally called phene. It is colourless liquid having characteristic odour.
Aromatic compounds contain high percentage of carbon and burn with sooty flame. They are cyclic compounds with alternate single and double bonds and are not attacked by normal oxidizing and reducing agents. Aromatic compounds do not undergo addition reactions easily and prefer substitution reaction.
STRUCTURE OF BENZENE
The molecular formula of benzene is C6H6. All six carbon atoms in benzene are sp2 hybridized. Benzene is a planar molecule with bond angle 120° each and all C-C bond lengths in benzene are equal to 139pm. Benzene has boiling point 353K. It is insoluble in water and soluble in alcohol, ether and chloroform. The structure of benzene is as follows:
AROMATIC CHARACTER (Huckel rule)
Huckel rule helps to predict whether a compound is aromatic or not. It states that any cyclic, planar and conjugated compound containing (4n+2)π electrons, where n=0,1,2,3,4… etc. is aromatic compound. Thus aromatic compounds have delocalized electron cloud of π electrons of 2,6,10,14 etc. e.g. Benzene (n=1) has 6π electrons. Naphthalene (n=2) has 10π electrons.
METHODS OF PREPARATION OF BENZENE
From acetylene (by cyclization)
When ethyne is passed through red hot iron quartz tube at 873K, it trimerises to give benzene.
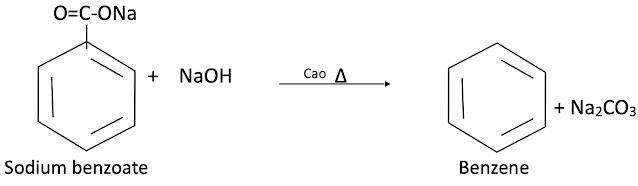
From sodium benzoate (by decarboxylation)
When anhydrous sodium benzoate is heated with soda-lime it gives benzene.
From phenol (By reduction)
When vapour of phenol passed over heated zinc dust, it gives benzene.
CHEMICAL PROPERTIES OF BENZENE
Addition reactions
Addition of chlorine to Benzene
Benzene when treated with chlorine in presence of bright sunlight or UV light, add up three molecules of chlorine to give benzene hexachloride.
Addition of hydrogen to Benzene
Benzene on heating with hydrogen gas in presence of catalyst like nickel at 453K, it adds up hydrogen molecules to give cyclohexane.
Substitution reaction
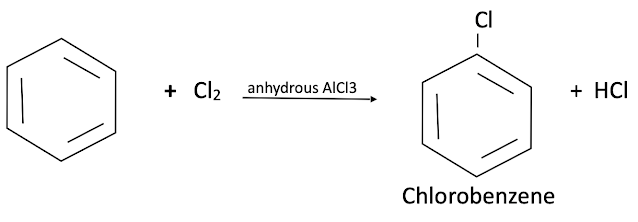
Halogenation of Benzene
Substitution of hydrogen atom of benzene by a halogen atom is called halogenation of benzene. Chlorine Or bromine react with benzene in dark in presence of Fe, FeCl3, AlCl3 or red phosphorus to give chlorobenzene Or bromobenzene.
Nitration of Benzene
Nitration is the substitution of - NO2 (nitro) group. When benzene is heated with a mixture of concentrated nitric acid and sulphuric acid in proportion 1:2 (nitrating mixture) at about 313K to 333K, it gives nitrobenzene.
Sulphonation of Benzene
Sulphonation is the substitution of - SO3H (sulphonic acid) group. When benzeblne is heated with fuming sulphuric acid (oleum) at 373K, it gives benzene sulphonic acid.
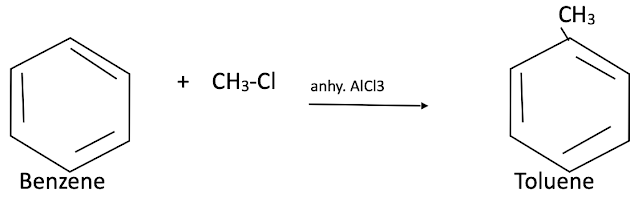
Friedel – crafts alkylation reaction of Benzene
When benzene is treated with an alkyl halide in the presence of anhydrous AlCl3 it gives alkyl benzene.
Friedel – crafts acylation reaction of Benzene
When benzene is heated with acyl halide or acid anhydride, in the presence of anhydrous AlCl3 , it gives corresponding acyl benzene.













%20(1).png)
