SI Table
Base physical quantity
|
Symbol for quantity
|
SI units name
|
SI units symbol
|
Length
|
l
|
Metre
|
m
|
Mass
|
m
|
Kilogram
|
Kg
|
Time
|
t
|
Second
|
s
|
Current
|
I
|
Ampere
|
A
|
Temperature
|
T
|
Kelvin
|
K
|
Amount of substance
|
n
|
Mole
|
Mol
|
Luminous intensity
|
Iv
|
candela
|
Cd
|
Prefixes for SI Units
Multiple
|
Prefix
|
Symbol
|
10-24
|
Yocto
|
y
|
10-21
|
Zepto
|
z
|
10-18
|
Atto
|
a
|
10-15
|
Femto
|
f
|
10-12
|
Pico
|
p
|
10-9
|
Nano
|
n
|
10-6
|
Micro
|
m
|
10-3
|
Milli
|
m
|
10-2
|
Centi
|
c
|
10-1
|
Deci
|
d
|
10
|
Deca
|
da
|
102
|
Hecto
|
h
|
103
|
Kilo
|
k
|
106
|
Mega
|
M
|
109
|
Giga
|
G
|
1012
|
Tera
|
T
|
1015
|
Peta
|
P
|
1018
|
Exa
|
E
|
1021
|
Zeta
|
Z
|
1024
|
yotto
|
Y
|
Mass:- amount of matter in object
=>
1 kg = 1000 gram
Weight: -force on the object by gravity.
Volume:- (length)3
=>
1 L = 1000 ml, 1000 cm3 = 1dm3
Density:- Amount of mass per unit volume.
=>
SI unit :- Kgm-3
Temperature:- It is the measurement of cooling or warming.
Three scales
=>
0C (Degree Celsius)
=>
0F degree Fahrenheit)
=>
k (Kelvin)
=>
freezing point of water = 0 0C
=>
Boiling point of water = 100 0C
Relationship
Or,
k = 0C +
273.15
Precision:- Closeness
of various measurement for same quantity.
Accuracy:- It is the
agreement of a particular value to the true value of the result.
Significant figure:-
The meaningful digits which are known with certainty.
SI Units - Basic Chemistry in Hindi Part 1
Watch this video at Youtube Link : https://youtu.be/eR6SCVLrVPE
by- www.ChemistryNotesInfo.com

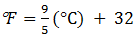
%20(1).png)
