Haloalkanes and Haloarenes Class 12th Chemistry Notes
These are organic chemistry study material chemistry notes on Haloalkanes and Haloarenes class 12 Chemistry for study and revision purpose of science students.
Haloalkanes and Haloarenes Class 12 Notes
Haloalkanes and Haloarenes Introduction
Alkanes are aliphatic saturated hydrocarbons having general formula CnH2n+2. While compounds that resemble (or which contain a benzene ring as a structural unit) are called as aromatic compounds or arenes.
When one or more hydrogen atoms at aliphatic hydrocarbon are replaced by corresponding number of halogen atoms (Chlorine, Bromine or Iodine) then compounds are called halogen derivatives of alkanes or haloalkanes.
e.g., Methyl Chloride (CH3Cl).
Similarly, when one or more hydrogen atoms of aromatic hydrocarbon are replaced by corresponding number of halogen atoms (Chlorine, Bromine or Iodine) then compounds are called halogen derivatives of arenes or haloarenes.
e.g., aryl halide.
Haloalkanes and haloarenes are used as solvent for relatively nonpolar compounds and are commercially important as dry-cleaning agents, refrigerants, propellants, drugs etc.
HALOALKANES – Haloalkanes and Haloarenes class 12
CLASSIFICATION OF HALOGEN DERIVATIVES OF ALKANES
1. Monohalogen Derivatives
When one hydrogen atom of alkane is substituted by one halogen atom, the compounds formed are called as monohydrogen derivatives of alkanes.
e.g. CH3Cl (methyl chloride)
CH3CH2Br (ethyl bromide)
2. Polyhalogen Derivatives
When more than one hydrogen atoms of alkanes are substituted by corresponding number of halogen atoms, the compounds formed are called polyhalogen derivatives of alkanes. They are further classified as
A] Dihalogen derivatives:- e.g. CH2Cl2
B] Trihalogen derivatives :- e.g. CHCl3
C] Tetrahalogen derivatives :- e.g. CCl4
ALKYL HALIDES
Alkyl halides are represented by the general formula CnH2n+1X or R-X where R = alkyl groups and X = halogen group. Alkyl halides are further classified into three categories on the basis of the type of the carbon atom to which the halogen atom is bonded.
1. Primary alkyl halides (1ᵒ) – general formula
R – CH2 – X
2. Secondary alkyl halides (2ᵒ) – general formula
3. Tertiary alkyl halides (3ᵒ) – general formula
PREPARATION OF ALKYL HALIDES
1. Preparation of Alkyl Halides from halogenations of alkanes
Alkyl chlorides are formed by the action of chlorine on alkanes in presence of diffused sunlight or ultraviolet light or at high temperature.
Ethane on chlorination forms ethyl chloride
CH3 – CH2 – H (Ethane) + Cl – Cl —–sunlight→ CH3 – CH2 – Cl (Ethyl Chloride) + HCl
The general formula for preparation of alkyl chloride is
R – H ( Alkane ) + Cl2 —–sunlight→ R – Cl ( alkyl Chloride )
Alkyl bromides are formed when alkanes are heated with bromine in the presence of anhydrous aluminium tribromide.
CH3 – H ( Methane) + Br – Br —-AlBr3→ CH3 – Br ( Methyl Bromide ) + HBr
Alkyl iodides are formed when alkanes are treated with iodine in the presence of oxidizing agent like HIO3, HgO, HNO3 etc.
5C2H6 (Ethane) + 2I2 + HIO3 → 5C2H5I (Ethyl Iodide) + 3H2O
Reaction of alkanes with fluorine is explosive and the product form hydrofluoric acid is poisonous and corrosive hence alkyl fluorides are not prepared by halogenations of alkanes.
2. Preparation of Alkyl Halides By addition of hydrogen to alkenes
Alkyl halides are obtained when hydrogen halide is treated with alkenes. In this reactions addition of hydrogen halide takes place across the double bond in alkene.
a) With symmetrical alkenes
CH2 = CH2 (Ethane) + HCl → CH – CH2Cl (Ethyl Chloride)
CH2 = CH2 (Ethane) + HBr → CH3 – CH2Br (Ethyl Bromide)
CH2 = CH2 (Ethane) + HI → CH3 – CH2I (Ethyl Iodide)
b) With unsymmetrical alkenes
The addition of hydrogen halides to an unsymmetrical alkene gives two products.
3. Preparation of Alkyl Halides From Alcohols
R – OH → R – X
-OH group of alcohol can be replaced by halogen using three types of reagent.
a) Reaction with hydrogen Halides
Alcohols react readily with hydrogen halides to yield alkyl halides and water.
1. Alkyl chlorides
C2H5OH ( Ethyl Alcohol ) + HCl ——-ZnCl2 ̶Δ→ C2H5Cl (Ethyl Chloride) + H2O
2. Alkyl Bromides
C2H5OH + HBr —-NaBr+H2SO4→ C2H5Br + H2O
Ethyl Alcohol reflux Ethyl bromide
3. Alkyl Iodide
C2H5OH (Ethyl Alcohol) + HI —Δ→ C2H5I (Ethyl iodide) + H2O
b) Reaction with phosphorous halides
i) With phosphorus trihalides (PX3)
Alkyl Chloride are prepared by refluxing alcohols with phosphorus trichloride
3C2H5OH (Ethyl alcohol) + PCl3 —Δ→ 3C2H5Cl (Ethyl Chloride) + H3PO4 (Phosphoric acid)
Alkyl bromide and alkyl iodides are prepared by the action of bromine or iodine in presence of red phosphorus on alcohols in situ.
Alkyl bromide and alkyl iodides are prepared by the action of bromine or iodine in presence of red phosphorus on alcohols in situ.
3C2H5OH (Ethyl Alcohol) + PBr3 → 3C2H5Br (Ethyl Bromide) + H3PO3 (Phosphoric acid)
ii) With phosphorous pentahalides (PX5)
Ethyl alcohol when refluxed with PCl5 forms ethyl Chloride
3C2H5OH (Ethyl alcohol) + PCl5 —Δ→ C2H5Cl (Ethyl Chloride) + HCl + POCl3 (Phosphorous-Oxychloride)
4. Preparation of Alkyl Halides By halogen exchange
Alkyl chloride or bromide when treated with NaI in presence of dry aceton give alkyl iodides. This reaction is known as FinKelstein reaction.
R – Cl + NaI → RI + NaCl
R – Br + NaI → RI + NaBr
e.g. C2H5Br + NaI → C2H5I + NaBr
PHYSICAL PROPERTIES OF ALKYL HALIDES
In this part of haloalkanes and haloarenes class 12 notes, we learn about physical properties of alkyl halides.
1. The lower members are gases at room temperature and higher members are liquid or solid.
2. Volatile halogen compound have sweet smell.
3. Boiling points of alkyl halides are greater than that of corresponding hydrocarbons. Boiling point increases with increase in molecular mass and force of attraction between molecules.
4. In case of isomeric haloalkanes, branching results in decrease in boiling point.
5. Alkyl halides are slightly soluble in water due to intermolecular forces of attraction and readily soluble in organic solvents.
5. Alkyl halides are slightly soluble in water due to intermolecular forces of attraction and readily soluble in organic solvents.
CHEMICAL REACTIONS OF ALKYL HALIDES
In this section we learn about different chemical reactions of alkyl halides.
A) Substitution reaction
The reaction in which an atom or a group of atoms are substituted (replaced) from substrate by same number of other atoms or groups are called substitution reaction.
R – X (Alkyl Halide) + Y– (Nucleophile) → R – Y + X– (Halide Ion)
1) Reaction with aq. KOH or NaOH
R – X (Alkyl Halides) + KOH (aq.) —boil→ R – OH (Alcohol) + KX
e.g. CH3 – I (Methyl Iodide) + KOH —boil→ C2H5OH (Ethanol) + KI
2) Reaction with ammonia (NH3)
R – X (Alkyl Halide alc.) + H – NH2 ̶̶̶̶ ̶ ̶ ̶ ̶ ̶ Δ̶̶ ̶ ̶ ̶ under pressure ̶ ̶ ̶ ̶ ̶→ R – NH2 (Primary Amine) + HX
e.g. CH3 – Cl + H – NH2 ̶̶̶̶ ̶ ̶ ̶ ̶ ̶ Δ̶̶ ̶ ̶ ̶ ̶ under pressure ̶ ̶ ̶ ̶→ CH3 – NH2 + HCl
3) Reaction with alc. KCN
R – X (Alkyl Halide) + KCN —boil→ R – CN (Alkyl Cyanide) + KX
e.g. C3H7Cl (2-Chloro-propane) + KCN —boil→ C3H7CN (2-Methyl-propane-nitrile) + KCl
4) Reaction with moist silver oxide
R – X (Alkyl Halide) + AgOH —moist AgO2 boil→ R – OH (Alcohol) + AgX
B) Elimination reactions
The reaction in which two atoms or group are removed from adjacent carbon atoms in a molecule to form an unsaturated compound is called an elimination reaction.
Dehydrohalogenation reaction
When alkyl halides are heated with alcoholic solution alkali hydroxide (KOH or NaOH), halogen atom from α-carbon atom and a hydrogen atom from adjacent β-carbon gets eliminated to form corresponding alkenes.
Saytzeff’s Rule
In a dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl group attached to doubly bonded carbon atoms.
C) Reaction with metals
1) Reaction with sodium : Wurtz reaction
When an alkyl halide is treated with sodium metal in pure and dry ether, a hydrocarbon is formed containing twice the number of carbon atoms present in the alkyl halide.
R – X (Alkyl Halide) + 2Na + X – R (Alkyl Halide) ̶ dry ether→ R – R (Alkane) + 2NaX (Sodium Halide)
e.g. Methyl bromide reacted with sodium in the presence of dry ether forms ethane.
e.g. Methyl bromide reacted with sodium in the presence of dry ether forms ethane.
CH3 – Br (Methyl Bromide) + 2Na + Br – CH3 (Methyl Bromide) —-dry ether→ CH3 – CH3 (Ethane) + 2NaBr
2) Reaction with magnesium : Formation of Grignard reagent
Grignard reagent is an organometallic compound in which the divalent magnesium is directly linked to an alkyl group and a halogen atom.
It is represented by general formula R – Mg – X. gringnard reagent can be prepared by reaction of alkyl halide with pure and dry magnesium in the presence of dry water.
R – X (Alkyl Halide) + Mg —dry ether→ R – Mg – X ( Alkyl magnesium halide) (Grignard reagent)
e.g. CH3 – I (Methyl Halide) + Mg dry ether→ CH3 – Mg – I (Methyl magnesium iodide)
Stereochemical aspects of Nucleophilic substitution reaction
Plan polarized light
When an ordinary light is passed through Nicol’s prism the light emerging out of it, consist of rays vibrating in one plane only. Such a beam of light which consist of the ways of light vibrating in one plane is called plane polarized light.
Optical activity
When plane polarized light is passed through solution of certain organic substances like glucose lactic acid etc. the plane of polarized light gets rotated through a certain angle. This property of phenomenon of certain organic substance of rotate the plane of polarized light towards right (clockwise) or towards left (anticlockwise) is called optical activity.
Optically active molecules
The molecules which can rotate the plane of plane polarized light are known as optically active molecules.
e.g. Glucose, Lactic acid, 2-chlorobutane etc.
This are of two types :
1) Dextro-rotatory molecules
Molecules which rotate the plane at polarization to the right hand side are known as dectro rotator molecules. These are denoted by (+) or d sign.
2) Laevo-rotatory molecules
These molecules rotate the plane of polarization to the left hand side are known as laevo molecules. These are denoted by (-) or l sign.
Optically inactive Molecules
The molecules which do not rotate the plane at plane polarized light are called optically inactive molecules.
Enantiomers
Stereo isomers which are non super imposable mirror images of each other and rotate the plane of plane polarized light through the same angle but in opposite direction are known as enantiomers.
Chirality
Any object which is non – super imposable with its mirror image is said to be chiral and this property is known as chirality.
REACTION MECHANISM
Mechanism of a reaction is a step by step description of exactly how the reactant are transformed into products in as much details as possible.
1) SN2 Mechanism
When the rate of nucleophilic substitution reaction depends upon the concentration of the two molecules, the substrate and the nucleophile (both the species), the reaction is said to be second order Nucleophilic substitution reaction. It is represented by SN2.
The rate of SN2 reaction is directly proportional to concentration of the substrate and nucleophilic.
e.g. CH3 – Br (Methyl Bromide) + OH– (nucleophilic) → CH3OH (Methanol) + Br–
e.g. CH3 – Br (Methyl Bromide) + OH– (nucleophilic) → CH3OH (Methanol) + Br–
Kinetic expression
: . Rate α [CH3Br] [OH–]
: . Rate = K [CH3Br] [OH–]
Where K is the specific constant.
2) SN1 Mechanism
When the rate of nucleophilic substitution reaction depends upon the concentration of only one molecule or species (the substrate) and not the nucleophile, the reaction is said to be first order Nucleophilic substitution reaction. It is represented by SN1.
The rate of SN1 reaction is directly proportional to concentration of only one substrate.
Kinetic expression
: . Rate α [(CH3)3C – Br]
: . Rate = K [(CH3)3C – Br]
Where K is the specific constant.
HALOARENES – Haloalkanes and Haloarenes Class 12 Notes
When one or more hydrogen atoms of an aromatic hydrocarbon are replaced or substituted by corresponding number of halogen atoms (such as chlorine, bromine or iodine), the new compound so obtained are called haloarenes.
Ar – H + Cl → Ar – Cl + H
They are represented as Ar – X, where Ar = Aryl Group (C6H5) and X = F,Cl, Br, I.
Classification of Haloarenes
Haloarenes are classified as mono, di or polyhalogen depending on whether they contain one, two or more halogen atoms in their molecules.
The C – X bond length in haloarenes is 1.70A. It is shorter in length and stronger than in alkyl halides having bond length 1.77A.
PREPARATION OF HALOARENES
1) Preparation of Haloarenes By electrophilic substitution.
Aryl Chlorides i.e. haloarenes can be easily prepared by electrophilic substitution reaction of arene with the chlorine (Cl2) and bromine (Br2) respectively in the presence of lewis acid catalysts like FeCl3 , Fe , BCl3 etc. e.g.
2) By SandMeyer’s reaction
When a primary aromatic amine, dissolved or suspended in cold aqueous mineral acid is treated with sodium nitrite a diazonium salt is formed. Mixing the solution of freshly prepared diazonium salt with Cuprous Chloride (Cu2Cl2) or Cuprous Bromide (Cu2Br2) result in the replacement of the diazonium group by –Cl or –Br. This reaction is known as Sandmeyer’s reaction. e.g.
CHEMICAL REACTION OF HALOARENES
This chemistry notes section of haloalkanes and haloarenes class 12, contain study material on chemical reactions of haloarenes.
1) Halogenation
Chlorobenzene reacts with chlorine in presence of anhydrous ferric chloride to give a mixture of ortho and para dichlorobenzene.
2) Nitration
When Chlorobenzene is heated with nitrating mixture (conc. Nitric acid + conc. Sulphuric acid) a mixture of 1 – chloro – 4 – nitrobenzene and 1 – chloro – 2 – nitro – benzene is formed.
3) Sulphonation
Chlorobenzene with conc. H2SO4 gives 2 – Chlorobenzene Sulfonic acid and 4- chlorobenzene Sulphonic acid.
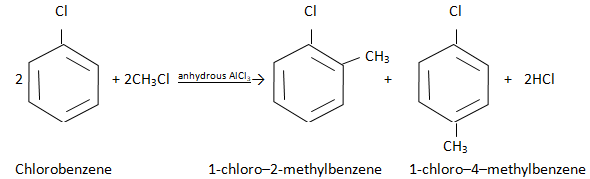
4) Friedel Craft’s alkylation reaction
5) Friedel Craft’s acylation reaction
6) Reaction with sodium metal: Wurtz fittig reaction
Aryl halide reacts with alkyl halides and undergoes coupling reaction when treated with sodium metal in the presence of dry ether to form alkyl benzene. This reaction is known as Wurtz Fittig Reaction.
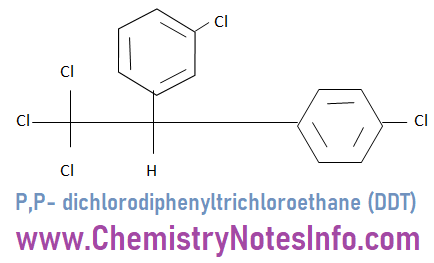
P,P- dichlorodiphenyltrichloroethane (DDT)
DDT is used as insecticide against malaria and it is used to kill various insects like housefly and mosquitoes.













%20(1).png)
