In these BSc MSc chemistry notes, we learn about Reactions of Aromatic Compounds. This is part 2 on reactions of aromatic compounds. This final part consists of following topics-
- Synthesis of Benzene Derivatives
- Benzylic Bromination
- Oxidation and Reduction of Substituted Benzenes
- The Gattermann Koch Formylation
- Nucleophilic Aromatic Substitution
- The Benzyne Mechanism
- Birch Reduction
- Side-Chain Reactions of Benzene Derivatives
- Reactions of Phenols
- Some uses of benzene derivatives…
Also read our part 1 notes i.e. Electrophilic Aromatic Substitution – Reaction of Aromatic Compounds (Part 1)
Reactions of Aromatic Compounds – BSc Chemistry Notes
These organic chemistry notes are very helpful for the BSc chemistry & MSc chemistry students. Now we learn about Reaction of Aromatic Compounds. So let’s start learning…
Synthesis of Benzene Derivatives
- To synthesize benzene derivatives with more than one substituent, we must always take into account the directing effects of each substituent.
- In disubstituted benzene, for example, the directing effects indicate which substituent must be added to the ring first.
- For example, the Br group in p-bromonitrobenzene is an ortho, para director and the NO2 group is a meta director.
Because the two substituents are para to each other, the ortho, para director must be introduced first when synthesizing this compound from benzene.
Synthesis of Benzene Derivatives – Reactions of aromatic compounds
- In pathway [1], bromination precedes nitration, yields the desired para product.
- In pathway [2], in which nitration precedes bromination, yields the undesired meta isomer.
- Pathway [1] yields both the desired para product as well as the undesired ortho isomer. Because these compounds are constitutional isomers, they are separable.
Pathway [1]: Bromination before nitration
Pathway [2]: Nitration before bromination
Synthesis of o-nitrotoluene from benzene.
Synthesis of o-nitrotoluene from benzene
- The CH3 group in o-nitrotoluene is an ortho, para director and the NO2 group is a meta director.
- Because the two substituents are ortho to each other, the ortho, para director must be introduced first.
- The synthesis thus involves two steps: Friedel–Crafts alkylation followed by nitration.
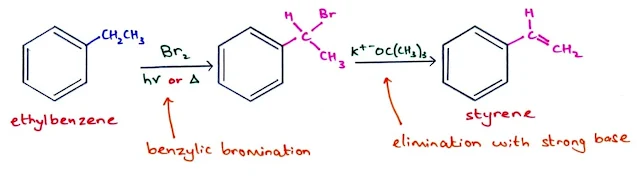
Halogenation of Alkyl Benzenes
- Benzylic C–H bonds (benzylic carbon bonded to the benzene ring) are weaker than most other sp3 hybridized C–H bonds, because homolysis forms a resonance-stabilized benzylic radical.
- As a result, alkyl benzene undergoes selective bromination at the weak benzylic C–H bond under radical conditions to form a benzylic halide.
- For example, radical bromination of ethylbenzene using either Br2 (in the presence of light or heat) or N-bromosuccinimide (NBS, in the presence of light or peroxides) forms a benzylic bromide as the sole product.
- The mechanism for halogenation at the benzylic position resembles other radical halogenations reactions, and so it involves initiation, propagation, and termination.
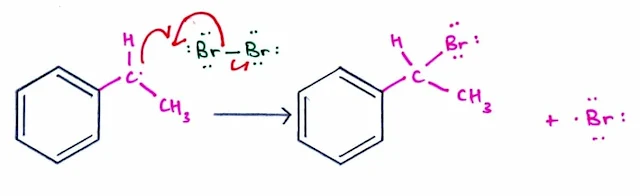
Mechanism: Benzylic Bromination
Step 1: Bond cleavage forms two radicals (Initiation).
- The reaction begins with homolysis of the Br – Br bond using energy from light or heat to form two Br• radicals.
Steps 2 and 3: One radical reacts and a new radical is formed (Propagation).
- Steps 2

- Steps 3

- Abstraction of a benzylic hydrogen by a Br• radical forms the resonance-stabilized benzylic radical in Step 2, which reacts with Br2 in Step 3 to form the bromination product.
- Because the Br• radical formed in Step 3 is a reactant in Step 2, Steps 2 and 3 can occur repeatedly without additional initiation.
Step 4: Two radicals react to form a bond (Termination).

- To terminate the reaction, two radicals, for example two Br• radicals, react with each other to form a stable bond.
- Thus, alkyl benzene undergoes two different reactions with Br2, depending on the reaction conditions.

- Ionic conditions:
- Br2 and FeBr3 are used as catalyst,
- Electrophilic aromatic substitution occurs, resulting in replacement of H by Br on the aromatic ring to form ortho and para isomers.
- Radical conditions:
- With Br2 and light or heat, substitution of H by Br occurs at the benzylic carbon of the alkyl group.
Synthesis of styrene from ethylbenzene.
The double bond can be introduced by a two-step reaction sequence: bromination at the benzylic position under radical conditions, followed by elimination of HBr with strong base to form the π bond.

Oxidation and Reduction of Substituted Benzenes – Reactions of Aromatic Compounds
- Oxidation and reduction reactions are plays important roles for preparing many other benzene derivatives.
Oxidation of Alkyl Benzenes
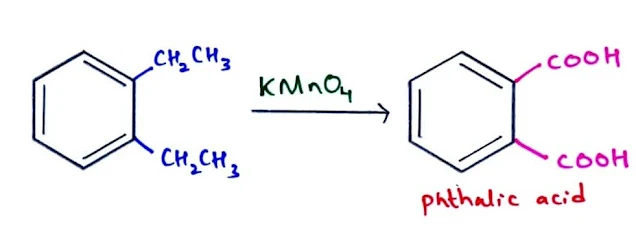
- Aromatic hydrocarbon such as arenes containing at least one benzylic C–H bond are oxidized with KMnO4 to benzoic acid, a carboxylic acid with the carboxy group (COOH) bonded directly to the benzene ring.
- Alkyl benzenes can also be oxidized to form benzoic acid.
- Oxidation results in the cleavage of carbon–carbon bonds.
- However, the final product has fewer carbon atoms than the starting material.

Note:
- Substituted benzene with more than one alkyl group is oxidized to dicarboxylic acids.
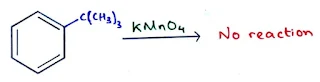
- Substituted benzene with no benzylic C–H bond is inert to oxidation.
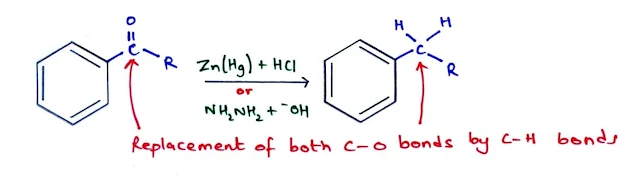
Reduction of Aryl Ketones to Alkyl Benzenes
- Ketones formed as the end products in Friedel–Crafts acylation can be reduced to alkyl benzenes by two different methods.
- The first method is the Clemmensen reduction whichuses zinc and mercury in the presence of strong acid.
- The second method is the Wolff–Kishner reduction whichuses hydrazine (NH2NH2) and strong base (KOH).
- The C=O bond in the starting material is converted to C–H bonds in the product.
- The reduction of aryl ketones is difficult and the reaction conditions must be harsh.
- There are two different ways to introduce an alkyl group on a benzene ring.
- The two methods are:
- Using Friedel–Crafts alkylation (one step method).
- Using Friedel–Crafts acylation to form a ketone, followed by reduction (two step method).
Two methods to prepare an alkyl benzene:
- Certain alkyl benzenes cannot be prepared directly by Friedel–Crafts alkylation method because of rearrangements.
- Therefore the two-step method (Friedel–Crafts acylation followed by reduction) is used to synthesize this type of compounds.
- For example propylbenzene cannot be prepared directly by a Friedel–Crafts alkylation. Propylbenzene can be prepared by a two-step procedure using Friedel–Crafts acylation followed by reduction.
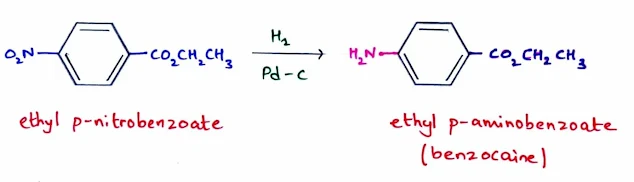
Reduction of Nitro Groups
- A nitro group (NO2) is easily introduced on a benzene ring by nitration with strong acid.
- The reduction of nitro groups is useful because it can be easily reduced to an amino group (NH2) under a variety of conditions.
- Nitro group can be reduced using H2 and a catalyst, or a metal (such as Fe or Sn) and a strong acid like HCl.
For example, reduction of ethyl p-nitrobenzoate with H2 and a palladium catalyst forms ethyl p-aminobenzoate, a local anesthetic commonly called benzocaine.
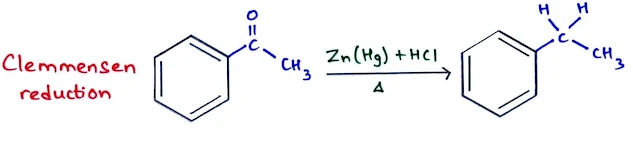
The Clemmensen Reduction: Synthesis of Alkylbenzenes
- Some alkybenzenes cannot be synthesized directly by Friedel-Craft alkylation reaction.
- For example, n-propylbenzene cannot be made by Friedel–Crafts alkylation.
- Therefore, we use the Friedel–Crafts acylation to make the acylbenzene, and then we reduce the acylbenzene to the alkylbenzene using the Clemmensen reduction.
- Clemmensen reduction: It is thetreatment of acylbenzene with aqueous HCl and amalgamated zinc (zinc treated with mercury salts).
- Benzene reacts with n-propyl chloride and to give isopropylbenzene, together with some diisopropylbenzene.
- Benzene reacts with propanoyl chloride and to give ethyl phenyl ketone (propiophenone), which is easily reduced to n-propylbenzene.
- The reagents and conditions for the Clemmensen reduction are similar to those used to reduce a nitro group to an amine.
- Carboxylic acids and acid anhydrides also serve as acylating agents in Friedel–Crafts reactions.
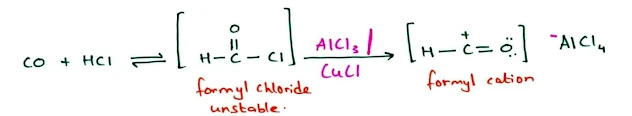
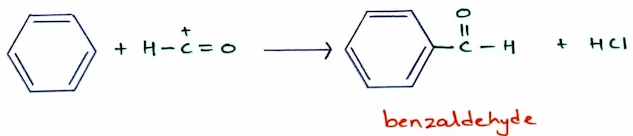
The Gatterman–Koch Formylation: Synthesis of Benzaldehydes
- We cannot add a formyl group to benzene by Friedel–Crafts acylation in the normal way.
- Because formyl chloride, which is unstable and cannot be bought or stored.
- Formylation can be accomplished by using a high-pressure mixture of carbon monoxide and HCl together with a catalyst consisting of a mixture of cuprous chloride (CuCl) and aluminum chloride.
- This mixture generates the formyl cation with a small concentration of formyl chloride.

- The reaction of formyl cation with benzene gives formyl benzene, also known as benzaldehyde.
- This reaction, called the Gatterman–Koch synthesis, is widely used in industry to synthesize aryl aldehydes.
Nucleophilic Aromatic Substitution – Reactions of Aromatic Compounds
- Nucleophiles can displace halide ions from aryl halides, particularly if there are strong electron-withdrawing groups ortho or para to the halide.
- Because a nucleophile substitutes for a leaving group on an aromatic ring, this type of reactions is called nucleophilic aromatic substitution.
- The following examples show that both ammonia and hydroxide ion can displace chloride from 2,4-dinitrochlorobenzene:
- Nucleophilic aromatic substitution is much more restrictive in its applications than electrophilic aromatic substitution.
- In nucleophilic aromatic substitution, a strong nucleophile replaces a leaving group such as a halide.
- The mechanism cannot be the SN1 or SN2 mechanism.
- The mechanism cannot be SN2 because aryl halides cannot achieve the correct geometry for backside displacement. Moreover the aromatic ring prevents the approach of the nucleophile to the back of the carbon bearing the halogen.
- The mechanism cannot be SN1 because strong nucleophiles are required for nucleophilic aromatic substitution, and the reaction rate is proportional to the concentration of the nucleophile. Thus, the nucleophile must be involved in the rate limiting step.
- Electron-withdrawing substituents (such as nitro groups) activate the ring toward nucleophilic aromatic substitution, suggesting that the transition state is developing a negative charge on the ring.
- Nucleophilic aromatic substitutions are difficult without a powerful electron-withdrawing group.
The Addition–Elimination Mechanism
- When hydroxide (the nucleophile) attacks the carbon bearing the chlorine, a negatively charged sigma complex results.
- The negative charge is delocalized over the ortho and para carbons of the ring and further delocalized into the electron-withdrawing nitro groups.
- Loss of chloride from the sigma complex gives 2,4-dinitrophenol, which is deprotonated in this basic solution.
Mechanism for Nucleophilic Aromatic Substitution (Addition–Elimination)
- The addition–elimination mechanism requires strong electron-withdrawing groups to stabilize a negatively charged sigma complex.
Step 1: A resonance-stabilized sigma complex is form when nucleophile attack the carbon bearing the chlorine.
Step 2: Loss of the leaving group gives the product.
Step 3: This product (a phenol) is acidic, and is deprotonated by the base.
- After the reaction is complete, acid would be added to re-protonate the phenoxide ion to give the phenol.
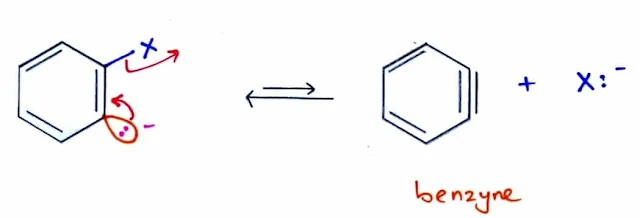
The Benzyne Mechanism: Elimination–Addition
- The addition–elimination mechanism for nucleophilic aromatic substitution requires strong electron-withdrawing substituents on the aromatic ring.
- Under extreme conditions, however, unactivated halobenzenes react with strong bases.
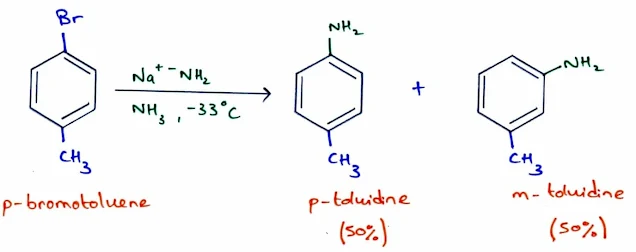
- For example, a commercial synthesis of phenol involves treatment of chlorobenzene with sodium hydroxide and a small amount of water in a pressurized reactor at 350 °C:
- Chlorobenzene can also react with sodium amide (an extremely strong base) to give aniline, Ph—NH2.
- This reaction does not require high temperatures; it can take place in liquid ammonia at -33°C.
- Nucleophilic substitution of unactivated benzene derivatives occurs by an elimination–addition mechanism, called the benzyne mechanism because of the unusual intermediate.
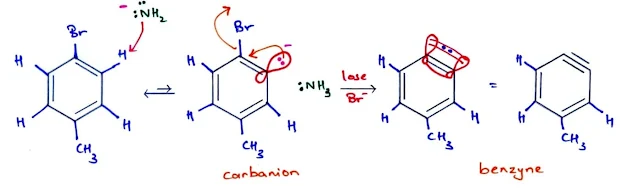
- Sodium amide or sodium hydroxide reacts as a base, by abstracting a proton.
- The product is
- A carbanion with a negative charge .
- A carbanion with a nonbonding pair of electrons localized in the sp2 orbital that once formed the C—H bond.
- The carbanion can remove a leaving group such bromide ion to become a neutral species.
- As bromide leaves with its bonding electrons, an empty orbital remains.
- This empty orbital overlaps with the filled orbital adjacent to it, forming additional bonding between these two carbon atoms.
- The two orbitals are directed 60° away from each other, so their overlap is not very effective.
- This reactive intermediate is called a benzyne because it can be represented by drawing a triple bond between these two carbon atoms.
- Triple bonds are usually linear, however, so this is a very reactive, highly strained triple bond.
- Amide ion (NH2–) is a strong nucleophile; it attacks at either end of the weak, reactive benzyne triple bond. Therefore protonation gives toluidine.
- About 50% of the product results from attack by the amide ion at the meta carbon, and about 50% from attack at the para carbon.
- The benzyne mechanism takes place when the halobenzene is unactivated toward nucleophilic aromatic substitution, and forcing conditions are used with a strong base.
- The benzyne mechanism consists of:
- Two step elimination forms a reactive benzyne intermediate.
- Nucleophilic attack, followed by protonation, gives the substituted product.
Nucleophilic Aromatic Substitution (Benzyne Mechanism)
- The benzyne mechanism (elimination–addition) takes place when the ring has no strong electron-withdrawing groups.
- It usually requires a strong base or high temperatures.
Step 1: Deprotonation adjacent to the leaving group gives a carbanion.
Step 2: The carbanion removes the leaving group to give a “benzyne” intermediate.
Step 3: The nucleophile attacks the reactive benzyme triple bond at either end.
Step 4: Reprotonation gives the product.
Aromatic Substitutions Using Organometallic Reagents
- Limitation of Friedel–Crafts alkylation:
- Rearrangements and multiple alkylations are common;
- The reaction fails on deactivated rings;
- It requires strong electrophiles such as AlCl3 and carbocations, which are incompatible with many functional groups.
- To avoid these limitations, organic chemists have developed new coupling reactions (reactions that form bonds) using a wide variety of methods that tolerate many other functional groups.
- Most coupling reactions use transition metals that change valences easily, adding and eliminating substituents as they pass from one oxidation state to another.
- Organocuprate reagents also couple with aryl and vinyl halides to make substituted benzenes and elongated alkenes.
- Recent research has developed many additional coupling methods using other transition metals in the reagents and catalysts.
- These reactions consist of palladium-catalyzed coupling reactions that form new carbon–carbon bonds at sp2 hybridized carbons like those in aromatic rings and in alkenes.
- The coupling reactions were developed by three famous scientist Richard F. Heck (University of Delaware), Ei-Ichi Negishi (Purdue University), and Akira Suzuki (Hokkaido University).
- Most of these reactions substitute organic groups for halogen atoms on aromatic rings or in alkenes.
- We use organocuprates to couple with aromatic rings and alkenes, and then use palladium-catalyzed reactions to form substituted aromatic rings.
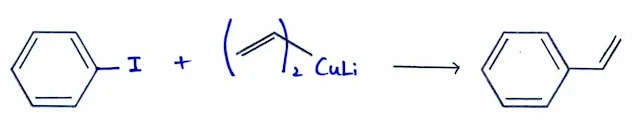
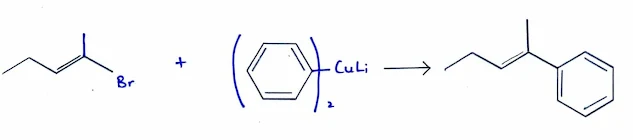
Couplings Using Organocuprate Reagents
- Lithium dialkylcuprate reagents (Gilman reagents) are formed by the reaction of two equivalents of an organolithium reagent with cuprous iodide.
- Reaction of the dialkylcuprate with an alkyl, aryl, or vinyl halide forms a new carbon–carbon bond.
1.Making the organolithium reagent:
2. Making the lithium organocuprate:

3. Coupling of the organocuprate with an alkyl, vinyl, or aryl halide:

- The mechanisms of these organocuprate reactions vary with the type of alkyl halide and organocuprate used.
- Organocuprate couplings do not occur via simple SN2 reactions, because these work well with sp2 hybrid substrates such as vinyl and aryl halides, which cannot undergo SN2 displacement.
- The aromatic ring can be present either in the aryl halide or in the dialkylcuprate reagent.
- Iodides, bromides, and chlorides are used as the halides.
Examples:
- An aryl halide with an alkyl or vinyl cuprate.
- A vinyl halide with an aryl cuprate, preserving the stereochemistry of the vinyl halide.
- An alkyl halide with an aryl cuprate.
- An acyl halide with an organocuprate, giving a ketone.
The Heck Reaction
- The Heck reaction is the palladium-catalyzed coupling of an alkene with an aryl or vinyl halide.
- A new C—C bond is formed at the less substituted end of the alkene, which is generally with trans stereochemistry.
The Heck Reaction
- In most cases, the halide is a bromide or an iodide, and the alkene is typically monosubstituted.
- The palladium catalyst might be Pd(OAc)2 or PdCl2 or a variety of other palladium compounds.
- Only a small amount of palladium catalyst is needed.
- A base such as triethylamine or sodium acetate is added to neutralize the HX released in the reaction.
- Many reactions use triphenylphosphine (PPh3) to complex with the palladium, which helps stabilize it and enhances its reactivity.
- The Heck reactions are used usually in drug synthesis, where the palladium catalysts can be recovered and recycled.
- Some Heck reactions use water as the solvent, which eliminates the purchase and disposal of hazardous organic solvents.
Examples of the Heck reaction:
- An aryl halide with an aryl olefin.
- An aryl halide with a conjugated acid or ester.
Addition Reactions of Benzene Derivatives – Reactions of Aromatic Compounds
Chlorination
- Aromatic compounds may undergo addition if forcing conditions are used.
- When benzene is treated with an excess of chlorine under heat and pressure (or with irradiation by light), six chlorine atoms add to form 1,2,3,4,5,6-hexachlorocyclohexane.
- This product is often called benzene hexachloride (BHC) because it is synthesized by direct chlorination of benzene.
- The addition of chlorine to benzene involves a free-radical mechanism, therefore the free- radical reaction are impossible to stop at an intermediate stage.
- The first addition destroys the ring’s aromaticity, and the next 2 moles of Cl2 add very rapidly.
- All eight possible stereoisomers are produced in various amounts.
- The most important isomer for commercial purposes is the insecticide lindane, which is used in a shampoo to kill head lice.
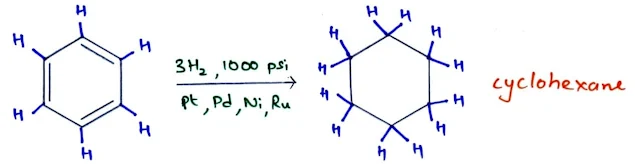
Catalytic Hydrogenation of Aromatic Rings
- Catalytic hydrogenation of benzene to cyclohexane takes place at high temperatures and pressures, often catalyzed by ruthenium or rhodium-based catalysts.
- Substituted benzenes react to give substituted cyclohexanes; disubstituted benzenes usually give mixtures of cis and trans isomers.
- Catalytic hydrogenation of benzene is the commercial method for producing cyclohexane and substituted cyclohexane derivatives.
- The reduction cannot be stopped at an intermediate stage (cyclohexene or cyclohexadiene) because these alkenes are reduced faster than benzene.
Birch Reduction
- Benzene derivatives are reduced to nonconjugated cyclohexa-1,4-dienes by treatment with sodium or lithium in a mixture of liquid ammonia and an alcohol.
- The Birch reduction provides a suitable method for making a wide variety of interesting and useful cyclic dienes.
- The mechanism of the Birch reduction is similar to the sodium/liquid ammonia reduction of alkynes to trans-alkenes.
- A solution of sodium in liquid ammonia contains solvated electrons that can add to benzene, forming a radical anion.
- The strongly basic radical anion abstracts a proton from the alcohol in the solvent, giving a cyclohexadienyl radical.
- The radical rapidly adds another solvated electron to form a cyclohexadienyl anion.
- Protonation of this anion gives the reduced product.
The Birch Reduction Mechanism
- The Birch reduction involves twice adding a solvated electron, followed by a proton, to the aromatic ring.
Preceding step: Formation of solvated electrons in the ammonia solution.
Steps 1 and 2: Addition of an electron, followed by a proton, forms a radical.
Steps 3 and 4: Addition of a second electron, followed by a proton, gives the product.
- The two carbon atoms that are reduced go through anionic intermediates.
- Electron withdrawing substituents stabilize the carbanions, while electron-donating substituents destabilize them.
- Therefore, reduction takes place on carbon atoms bearing electron withdrawing substituents (such as those containing carbonyl groups) and not on carbon atoms bearing electron-releasing substituents (such as alkyl and alkoxyl groups).
- A carbon bearing an electron-withdrawing carbonyl group is reduced
- A carbon bearing an electron-releasing alkoxyl group is not reduced
- Substituents that are strongly electron-releasing (-OCH3, for example) deactivate the aromatic ring toward Birch reduction.
- Lithium is often used with these deactivated systems, together with a cosolvent (often THF) and a weaker proton source (tert-butyl alcohol).
- The stronger reducing agent, combined with a weaker proton source, enhances the reduction.
Side-Chain Reactions of Benzene Derivatives – Reactions of Aromatic Compounds
- Many reactions are not affected by the presence of a nearby benzene ring; however others depend on the aromatic ring to promote the reaction.
- For example, the Clemmensen reduction is occasionally used to reduce aliphatic ketones to alkanes, but it works best reducing aryl ketones to alkylbenzenes.
Permanganate Oxidation
- An aromatic ring gives extra stability to the nearest carbon atom of its side chains.
- The aromatic ring and one carbon atom of a side chain can survive a vigorous permanganate oxidation.
- The product is a carboxylate salt of benzoic acid.
- Hot chromic acid can also be used for this oxidation.
- Permanganate oxidation is useful for making benzoic acid derivatives, as long as any other functional groups are resistant to oxidation.
- Functional groups such as -NO, halogens, -COOH, and –SO3H usually survive this brutal oxidation.
Side-Chain Halogenation
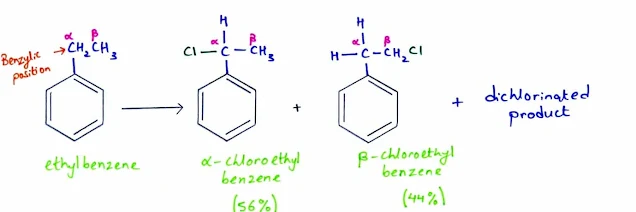
- Alkylbenzenes undergo free-radical halogenation much more easily than alkanes because abstraction of a hydrogen atom at a benzylic position gives a resonance stabilized benzylic radical.
- For example, ethylbenzene reacts with chlorine in the presence of light to give α-chloroethylbenzene
- Further chlorination can occur to give a dichlorinated product.
- Chlorination shows a preference for α substitution (the α position is the benzylic carbon bonded to the benzene ring), but the chlorine radical is too reactive to give entirely benzylic substitution. Therefore mixtures of isomers are often produced.
- In the chlorination of ethylbenzene, for example, there is a significant amount of substitution at the β carbon.
- Bromine radicals are not as reactive as chlorine radicals, so bromination is more selective than chlorination.
- Bromine reacts exclusively at the benzylic position.

- Elemental bromine (much cheaper) or N-bromosuccinimide may be used as the reagent for benzylic bromination.
- N-Bromosuccinimide is preferred for allylic bromination because Br2 can add to the double bond.
Reactions of Phenols – Reactions of Aromatic Compounds
- Phenols can be acylated to give esters.
- Phenoxide ions are able to serve as nucleophiles in the Williamson ether synthesis.
- The formation of phenoxide ions is very easy because phenols are more acidic than water.
- Aqueous sodium hydroxides can deprotonates phenols to give phenoxide ions.
Oxidation of Phenols to Quinones
- Phenols undergo oxidation and can produced different types of products from those seen with aliphatic alcohols.
- Chromic acid oxidation of a phenol gives a conjugated 1,4-diketone called a quinone.
- Phenols compounds can slowly autoxidise to dark mixtures containing quinines in the presence of air.
- Hydroquinone (benzene-1,4-diol) is easily oxidized because it already has two oxygen atoms bonded to the ring.
- Weak oxidants like silver bromide (AgBr) can oxidize hydroquinone very easily.
- Silver bromide is reduced to black metallic silver in a light sensitive reaction.
- Any grains of silver bromide that have been exposed to light react faster than unexposed grains.
Principle of photography
- Black-and-white photography is based on this reaction.
- A film containing small grains of silver bromide is exposed by a focused image.
- Where light strikes the film, the grains are activated.
- The film is then treated with a hydroquinone solution (the developer) to reduce the activated silver bromide grains, leaving black silver deposits where the film was exposed to light.
- The result is a negative image, with dark areas where light struck the film.
Electrophilic Aromatic Substitution of Phenols – Reactions of Aromatic Compounds
- Phenols are highly reactive substrates for electrophilic aromatic substitution because the nonbonding electrons of the hydroxyl group stabilize the sigma complex formed by attack at the ortho or para position.
- Therefore, the hydroxyl group is strongly activating and ortho, para-directing.
- Phenols are excellent substrates for halogenation, nitration, sulfonation, and some Friedel–Crafts reactions.
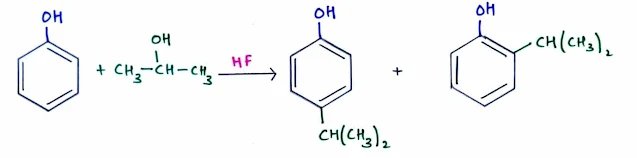
- Because they are highly reactive, phenols are usually alkylated or acylated using relatively weak Friedel–Crafts catalysts (such as HF) to avoid overalkylation or overacylation.
- Phenoxide ions can be formed by treating a phenol with sodium hydroxide.
- Phenoxide ions are more reactive than phenols toward electrophilic aromatic substitution.
- Because they are negatively charged, phenoxide ions react with positively charged electrophiles to give neutral sigma complexes whose structures resemble quinones.
- Phenoxide ions are strongly activated and can undergo electrophilic aromatic substitution with a weak electrophile sach as carbon dioxide.
- The carboxylation of phenoxide ion is an important industrial synthesis of salicylic acid, which is then converted to acetylsalicylic acid (aspirin).
Thanks for your interest in Chemistry Notes Info chemistry notes. If you like these notes then Share these notes with your friends…
Our other MSc – BSc College Chemistry Notes are given below-
- Mathematical Concepts
- Zinc Metalloenzymes
- Atomic Structure
- Thermodynamics
- Solid State Chemistry
- Chemistry of Elements of First Transition Series
- Iron Metalloporphyrins Complexes in Bioinorganic Molecules
- Electromagnetic spectrum UV and Visible spectroscopy
- Electrophilic Aromatic Substitution – Reaction of Aromatic Compounds




























































%20(1).png)
