SOME BASIC PRINCIPLES AND TECHNIQUES IN ORGANIC CHEMISTRY
Organic chemistry is the chemistry of carbon compounds except oxides of carbon and metal carbonates. The term organic literally means derived from living organism. All organic compounds contain carbon as their essential constituent. Carbon atom has unique property to form bonds with other carbon atoms. This property of forming bonds with atoms of the same element is called catenation.
Carbon form strong bonds with many other elements and especially with other carbon atoms to form chains and rings that gives rise to millions of organic compounds.
CLASSIFICATION OF ORGANIC COMPOUNDS
Organic
compounds are broadly classified in two ways.
1] Based on carbon skeleton
Aliphatic compounds
Aliphatic compounds are the compounds in which carbon atoms are joined to form an open chain. Their
structure may consist of straight chain or branched chain.
e.g. CH3 – CH2 – CH2 – CH3
Cyclic compounds
These
are the compounds in which carbon atoms are joined to form one or more rings.
They are further classified into two types.
a] Homocyclic or carbocyclic
In
these compounds, the ring is made up of carbon atoms only. They are further
divided into two types.
i) Alicyclic compounds
These compounds show some of the properties similar to those of aliphatic compounds. Carbon atoms are linked by single bonds only.
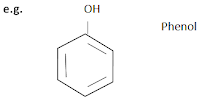
ii) Aromatic compounds
Aromatic compounds are the compounds which contains at least one aromatic ring which resembles benzene in their chemical behaviour.
b] Heterocyclic compounds
These
compounds include one or more heteroatoms like O, N, S etc. in the ring of
carbon atoms. They are also divided into two types.
i) Hetero – alicyclic compounds
Alicyclic
compounds which contain at least one heteroatom in the ring are called hetero-alicyclic
compounds.
ii) Hetero – aromatic compounds
Aromatic compounds which contain at least one heteroatom
in the ring are called hetero – aromatic compounds.
2] Based on functional group
An atom or a group of atoms in the
organic molecule which determines its characteristic chemical properties is
called the functional group.
e.g.
– OH, - X, - CHO, - COOH, - NH2,
etc. are functional group. The chemistry of every organic molecule is
determined by the functional group it contains.
Homologous series
A
series of organic compounds each containing a characteristic functional group,
and the successive members differ from each other in molecular formula by a –CH2–
(methylene) group is called homologous series.
PURIFICATION OF ORGANIC COMPOUNDS
The
purification of organic compounds involves the following processes.
1. Crystallization
This
is the most common method used to purify organic solids which dissolve in
particular solvent. The purification is done on the basis of differences in
solubility of a given organic compound and impurities.
2. Sublimation
It
is a process which is used for solids which directly change into vapor state
upon heating without passing the liquid state and vapors on cooling give back
the solid Substance. Impure samples of naphthalene, anthracene, camphor, etc. are
purified by this method.
3. Distillation
The
process in which liquid is converted into its vapor phase at its boiling point
and the vapors is then condensed back to liquid on cooling is known as
distillation. This method is used if the organic liquid is stable at its
boiling point and it contains non – volatile impurities.
4. Fractional distillation
The
process of separating and purifying the components of a mixture of two or more
miscible liquids having different boiling points is known as fractional
distillation. The liquid which is more volatile distills out first leaving
behind the less volatile liquid in the distillation flask.
5. Steam distillation
Liquids
which are immiscible with water but are steam volatile are separated by this
technique. Aniline, nitrobenzene, bromobenzene etc. can be steam distilled.
6. Fractional crystallization
The
process of separating the components of a mixture of two or more solids, having
different solubilities in the same solvent at the same temperature, by step –
wise crystallization is known as fractional crystallization.
7. Differential extraction
The
organic solvents like benzene, chloroform, petroleum ether etc. are immiscible
with water such solvents are used to extract an organic compound present in
aqueous solution by the method of differential extraction.
8. Chromatography
Chromatography
is the technique used for the separation, isolation, purification and
identification of constituents of a mixture. This technique depends on the
distribution of the mixture between two phase, one stationary phase and other
moving or mobile phase. Depending upon the principle involved, it is divided
into two types.
a) Adsorption chromatography
It
is based on the principle of differential adsorption. Different compounds are
adsorbed on adsorbent to different degrees. It also has two types i) column
chromatography and ii) thin – layer chromatography (TLC).
b) Partition chromatography
This
technique is based on continuous differential partitioning of components of a
mixture between stationary and mobile phases.
Determination of empirical formula
The
formula of a compound which gives the simple whole number ratio of the atoms of
various elements present in one molecule of the compound is called its empirical
formula. It involves following steps.
Step – I
The
percentage composition of elements presents in a given compound is determined by
quantitative analysis.
Step – II
The
percentage of each element is divided by the respective atomic mass. This is
giving the atomic ratio of constituent's atoms present in a given compound.
Step – III
The
simplest ration is found by dividing all the atomic ratios by the smallest atomic
ratio.
Molecular formula
The
formula which gives the actual number of atoms of various elements present in
one molecule of the compound is called its molecular formula. Molecular
formula is determined from empirical formula as –
Molecular
formula = n x empirical formula
Where
n is whole integer given by-
ISOMERISM
The compounds having same molecular formula and different structural
formulae are called isomers of each other. This phenomenon is known as
isomerism.
It has two main types.
1. Stereoisomerism
The
stereoisomers have the same structural formula but differ in the arrangement of
atoms or groups in space.
2. Structural isomerism
The
structural isomers differ from each other in the arrangement of the atoms or
groups within the molecules.
It
can be further divided as:
a) Chain or nuclear isomerism
It
is the type of isomerism in which the isomers differ in the chain of carbon
atoms. For example, C4H10 has two isomers.
b) Position isomerism
It
is the type of isomerism in which the different isomers differ in the position
of the functional group. For example, C3H7OH has two
isomers.
c) Functional isomerism
It is the type of the isomerism in which isomers have different functional group. For example, C2H6O has two isomers:
d) Metamerism
It
is the type of isomerism in which the isomers differ in the nature of alkyl
group attached to the same functional group. For example, C5H10O
has two isomers.
C2H5
– CO – C2H5 (Diethyl
Ketone) and CH3 – CO – C3H7
(Methyl propyl Ketone)
FUNDAMENTAL CONCEPTS IN ORGANIC REACTION MECHNAISM
Electron displacements in covalent bonds
Inductive effect
When an organic molecule has a polar covalent bond in its structure
polarity is induced in adjacent carbon – carbon single bond too. This is called
inductive effect. The arrow head put in the center of the bond is used to
represent the inductive effect. The direction of arrow head indicates the
direction of permanent electron displacement. For example,
This
effect decreases rapidly as the length of carbon chain increases. There are two
types of inductive effect.
i) – I effect
Atoms
or group of atoms which are highly electronegative or carry positive charge are
electron – withdrawing groups and such groups are said to have – I
effect.
e.g.
– F, - Cl, -Br, - I , - NO2, - CN, - COOH, - COOR, - SO3H,
etc.
ii) + I effect
Atoms
or group of atoms which are electropositive or carry negative charge are
e.g.
Metals like Na, K, Mg, Zn etc. and alkyl groups such as – CH3, - CH2CH3,
- CH(CH3)2, - C(CH3)3, - C(C2H5)3,
etc.
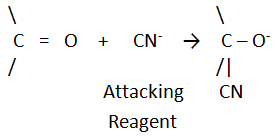
Electromeric effect
The complete transference of shared pair of electrons to one of the atom
joined by a multiple bond in presence of an attacking reagent this is known as
electromeric effect.
This is a temporary effect and takes place only in the presence of an attacking reagent. For example:
Resonance effect
The
polarity developed within a molecule is due to the interaction of two ∏ - bonds
or a ∏ - bonds with the lone pair of electrons present on the adjacent atom is
called the resonance effect or mesomeric effect. It is a permanent
effect and depending, upon the direction of transfer of electrons it is of two
types.
i) +R effect
When
the transfer of electron is away from an atom or substituent group attached to
the conjugated system, it is termed as +R effect. For example +R effect in
aniline.
ii) – R effect
When
the transfer of electron is towards the atom or substituent group attached to
the conjugated system, it is termed as – R effect. For example – R effect in nitrobenzene.
Hyper conjugation
The delocalization of electrons due to overlap between a P – orbital and
sigma (σ) bond of C – H is called hyper conjugation or no bond resonance. Greater the number of
alkyl groups attached to a positively charged carbon atom, greater is the hyper
conjugation interaction and greater is the stability of cation. Thus the
relative stability of cation decreases in the order.
(CH3)3C+
> (CH3)2CH+ > CH3CH2+
> CH3+
Bond fission
In
any chemical reaction, when a reactant is converted into products one or more
bonds in the reactant are broken and new bonds are formed. The process of
breaking or cleavage of covalent bond is known as bond fission. The bond
fission takes place in two ways.
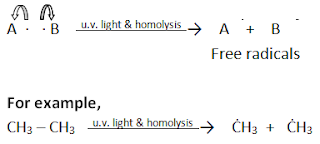
i) Hemolytic fission
The
symmetrical breaking of a covalent bond between two atoms such that each atom acquires
one electron of the shared pair is called hemolytic fission or homolysis.
Such
fission takes place in the presence of ultraviolet light or high temperature.
The cleavage of a bond results in the formation of free radicals. A free
radical is a neutral species (atom or group) which contain an unpaired
electron. A hemolytic fission is represented as:
ii) Heterolytic fission
The
unsymmetrical breaking of a covalent bond between two atoms such that the more electronegative
atom acquires both the electrons of the shared pairs is called heterolytic
fission or heterolysis .
Such a fission takes place in the presence of polar solvent. The cleavage of a bond results in the formation of ions. This ions formed are unstable and reactive. One of the ion has a positive charge called as cation and the other ion has at least one lone pair and a negative charge called as an anion. Organic reactions which proceed by heterolysis are called ionic or heteropolar simply polar reaction. A heterolytic fission is represented as:
The
species in which carbon atom passes a positive charge is called carbanion and
the species in which carbon atom possesses negative charge is called carbanion.
Example
of heterolysis is,
Reaction intermediates
i) Free radicals
An uncharged species which is electrically neutral and contains a single
electron is called free radical.
Free
radical is highly reactive and therefore has a transitory existence i.e. it is short
– lived. The stability of free radicals decreases in the order triphenyl methyl
> benzyl > allyl > 3ᵒ > 2ᵒ > 1ᵒ > methyl > vinyl.
ii) Carbocation
A species in which carbon atom bears a positive charge is called a carbocation.
Positively
charged carbon is sp2 hybridized. It is electron – deficient. It is
Lewis acid and act as an electrophile. It has planer geometry.
e.g.
tetra – butyl carbocation (CH3)3C+.
iii) Carbanion
A
species in which carbon atom bears a negative charge is called a carbanion.
Negatively charged carbon is Sp3 / Sp2 hybridized. It is
electron – rich. It is Lewis base and act as a nucleophile. It has pyramidal
geometry.
iv) Carbenes
The
highly reactive intermediate containing neutral and divalent carbon atoms is
called carbene.
For example, CH2
Types of attacking reagent.
1) Electrophiles
Electrophiles
are electron – deficient species. They are either positively charge species
like H+, NO+2 etc. or molecules having
incomplete octet of electrons like BF3, AlCl3, ZnCl2
etc.
Since
electrophiles are electron deficient they accept a pair of electrons from donor
atoms and thus they are electron loving reagents. All electrophiles are
basically lewis acids.
2) Nucleophiles
Nucleophiles
are electron rich species. They are either negatively charged species like OH-,
CN-, Cl-, Br-etc. or molecules containing at
least one lone pair of electrons on central atom like H2O, NH3,
H2S, R-OH, R-NH2, etc. since nucleophiles are electron
rich, they donates pair of electrons to acceptor atoms and thus they are
nucleus loving reagents. All nucleophiles are Lewis base.
Types of organic reactions
1) Substitution reaction
A
reaction in which an attacking species replaces another atom or group in
substrate is called substitution reaction.
e.g.
2) Addition reaction
When
two molecules combine to form one product molecule, it is called an addition
reaction.
e.g.
CH2
= CH2 (Ethylene) + HCl
→ CH3 – CH2
– Cl (Ethyl chloride)
3) Elimination reaction
When
one molecule is split into two fragment molecules, it is called an elimination
reaction.
e.g.
4) Rearrangement reaction
A
reaction in which either the carbon skeleton or the functional group or both
are modified is known as rearrangement reaction.
e.g.
Organic chemistry class 12 conversion questions with answers
Dear Science Students! Here are some organic chemistry conversion questions for Class 12 with answers:
1. Convert Ethanol to Ethanoic Acid:
Answer: Ethanol can be converted to ethanoic acid by oxidizing it using an oxidizing agent like potassium dichromate (K2Cr2O7) and sulfuric acid (H2SO4).
Equation:
CH3CH2OH + [O] → CH3COOH + H2O
2. Convert Benzene to Nitrobenzene:
Answer: Benzene can be converted to nitrobenzene through nitration using a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4).
Equation:
C6H6 + HNO3 → C6H5NO2 + H2O
3. Convert Propanone to Propene:
Answer: Propanone (acetone) can be converted to propene through the dehydrogenation process using a strong base like potassium hydroxide (KOH).
Equation:
(CH3)2CO → CH2=CH2 + H2O
4. Convert Toluene to Benzyl Alcohol:
Answer: Toluene can be converted to benzyl alcohol through reduction using a reducing agent like sodium borohydride (NaBH4).
Equation:
C6H5CH3 + NaBH4 → C6H5CH2OH + NaBO2 + H2
5. Convert Ethylamine to Acetamide:
Answer: Ethylamine can be converted to acetamide through the reaction with acetic anhydride.
Equation:
CH3COOC2H5 + NH2CH2CH3 → CH3CONHC2H5 + CH3COOH
6. Convert Butanal to Butanoic Acid:
Answer: Butanal can be converted to butanoic acid through oxidation using an oxidizing agent like potassium dichromate (K2Cr2O7) and sulfuric acid (H2SO4).
Equation:
CH3CH2CH2CHO + [O] → CH3CH2CH2COOH
7. Convert Ethene to Ethanol:
Answer: Ethene can be converted to ethanol through hydration, typically in the presence of a catalyst like sulfuric acid (H2SO4).
Equation:
CH2=CH2 + H2O → CH3CH2OH
8. Convert Ethylbenzene to Benzoic Acid:
Answer: Ethylbenzene can be converted to benzoic acid through oxidation using potassium permanganate (KMnO4) and sulfuric acid (H2SO4).
Equation:
C6H5CH2CH3 + [O] → C6H5COOH + H2O
These conversion reactions are common examples in organic chemistry and are frequently encountered in Class 12 syllabus. Remember to balance the chemical equations and pay attention to reaction conditions and reagents used in each conversion.
Points to be remember from organic Chemistry
- Atoms Stick Together: In organic chemistry, things called "atoms," like carbon and hydrogen, like to share their outer parts with each other, forming bonds. This sharing is called "covalent bonding."
- Carbon's Special Trait: Carbon is like a friendly glue because it can make four bonds with other atoms. This makes it the building block for lots of different organic compounds.
- Different Arrangements: Organic compounds can look different even if they have the same ingredients. This is because atoms can be arranged in various ways.
- Special Groups Matter: Some groups of atoms, like OH or CO, give organic compounds their special qualities. These groups are called "functional groups."
- Naming Rules: There are rules for naming organic compounds, like calling them by specific names using the IUPAC system.
- Naming Different Shapes: Compounds that look similar but are arranged differently need different names too.
- Shifting Electrons: Sometimes, electrons in compounds move around, creating different versions of the same compound. This is called "resonance."
- Acids and Bases: Some compounds can be sour (acidic), and some can be bitter (basic). Knowing which ones which are is important.
- How Things Change: Learn how organic compounds transform during reactions, like switching one piece for another.
- Left and Right-Handed: Some molecules can come in two versions that are like mirror images. These are called "left-handed" and "right-handed."
- Polarity Matters: Think of molecules like magnets – some are attracted to water, and some aren't. This affects how they dissolve.
- Cleaning Up: Scientists have ways to clean up messy mixtures of compounds, like using special tools to separate them.
- Using Light and Machines: Special tools like flashlights (infrared spectroscopy), magnets (NMR spectroscopy), and scales (mass spectrometry) help scientists understand compounds better.
- How Reactions Happen: Find out how different reactions work, like what needs to be added and what conditions they like.
- Inside the Reaction: Learn what happens at the tiny level during a reaction, including things that form in the middle.
- Who's Who in Reactions: Some compounds like to take electrons (called electrophiles), while others like to share (called nucleophiles).
- Switching Groups: Discover how different parts of molecules can be changed into other parts through different reactions.
- Making New Stuff: Organic chemistry is like building with blocks – you can make new things by putting pieces together in special ways.
- Staying Safe: When working with chemicals, always be safe. Wear the right gear and follow safety rules in the lab.
- Practice Makes Perfect: Keep practicing problems and learning about how things react to get better at organic chemistry.

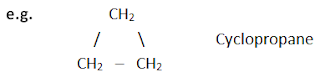
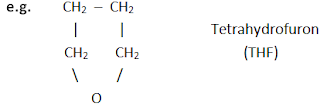


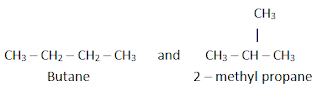
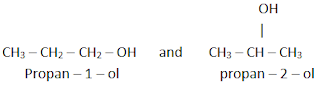
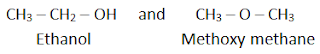
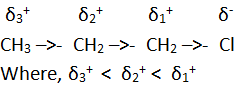



%20(1).png)

Thanks you so much sir for organic chemistry notes.
ReplyDelete