Redox Reactions Class 11th Chemistry Notes
Chemical reactions are generally
classified into three types:
1) Precipitation reactions
2) Acid – base neutralization
3) Redox reactions
Redox reaction is a very important
group of reactions. These reactions are involved in large number of processes
in nature, biological and industrial.
Definition of Redox Reaction
Don't recollect it yet, don't worry !!! just Learn more about Redox Reactions Class 11th Notes below→
Video on Redox Reaction Class 11th Chemistry Notes
CLASSICAL CONCEPT OF OXIDATION AND
REDUCTION
Oxidation is a reaction which involves the
addition of oxygen or any other electronegative element or the removal of
hydrogen or any electropositive element from the substance.
For example,
C(s) + O2(g) → CO2(g) (oxidation of carbon)
Reduction reaction involves the addition of
hydrogen or any electropositive element or removal of oxygen or any electronegative
element. It is just the reverse of oxidation.
For example,
Cl2 + H2 →
2HCl (reduction of Cl)
ELECTRONIC CONCEPT OF OXIDATION AND
REDUCTION
OXIDATION
An oxidation is defined as the loss
of one or more electron from a substance.
For example,
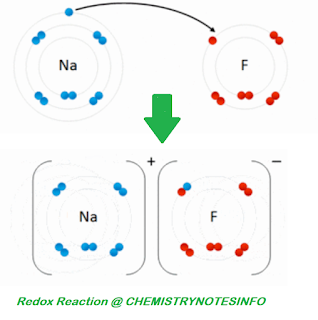
Na → Na+ + e-
REDUCTION
A reduction is defined as the gain of
one or more electrons by a substance.
For example,
Cl2 + 2e- → 2Cl-
OXIDIZING AGENT OR OXIDANT
Oxidizing agent is a substance which
accepts or gain electrons and causes the oxidation of other substance.
REDUCING AGENT OR REDUCTANT
Reducing agent is a substance which
donates electrons and causes reduction of other substance.
REDOX REACTION (oxidation – reduction
reaction)
The reactions in which oxidation and
reduction reactions occur simultaneously are called redox reaction.
In redox reactions, electrons are
transferred from one substance to the other substance, therefore these
reactions are electron transfer reactions.
For example,
Loss of 2e- (oxidation)
Zn
+ Cu2+ →
Zn2+ + Cu
Gain of 2e- (Reduction)
OXIDATION NUMBER OR OXIDATION STATE
Oxidation number of an element in a
compound is defined as the number of electrical charges it carries when all
other atoms are removed from it as ions.
Rules to assign oxidation numbers
1) The oxidation number of an
atom in free uncombined elemental state is zero.
For example,
H2, Ca, Cl2,
O3, S8, P4, and so on has oxidation number
zero.
2) The oxidation number of an
atom in a monoatomic ion is equal to its charge.
For example,
K+ Ba2+ Cr3+ Br- S2-
↑ ↑
↑ ↑ ↑
+1 +2
+3 -1 -2
3) When hydrogen atom is
bounded to non – metals, its oxidation number is +1. And when it is bounded to
metals, its oxidation number is -1.
For example,
[O – H]- H – O – H
Li – H
H – Ca – H
-2 +1
+1 -2 +1
+1 -1
-1 +2 -1
4) The oxidation number of
oxygen is usually -2 in all of its compounds except in peroxide or peroxide ion
where it has oxidation number of -1.
For example,
Ca – O H – O – O – H [O – O]2-
+2 -2
+1 -1 -1 +1
-1 -1
5) The oxidation number of F
is -1 in all of its compounds. The other halogens Cl, Br and I usually exhibit
oxidation number of -1 in their halide compounds. However when Cl, Br and I are
bonded to oxygen then they exhibit oxidation number of +1.
For example,
H – F K – Br Cl – O – Cl H – O – Cl
+1 -1 +1
-1 +1 -2
+1 +1 -2
+1
6) The algebraic sum of the
oxidation number of all atoms in a neutral molecule is zero and for the
polyatomic ion it is equal to net charges of the ion.
For example,
In H – Cl
Oxidation number of H +
oxidation number of Cl = 0
= +1
-1 = 0
Using
above rules we can calculate oxidation number of an atom in any molecule or ion
Problems
Determine the oxidation number of
a) N in HNO3
HNO3 is neutral
molecule
: . Sum of oxidation number of all atom of HNO3
is equal to zero.
[ O.N. of H ] + [
O.N. of N ] + [ 2 X O.N. of O ] = 0
= +1
+ [O.N. of N ] + [ 2
X (-2) ]
= 0
= +1
+ [O.N. of N ] -
4 = 0
= O. N
of N - 3
= 0
O. N
of N = +
3
: . Oxidation number of N in HNO3 is
+3.
b) Pt in PtCl62-
PtCl62- is ionic species.
: . Sum of oxidation number of all atom of PtCl62- is equal to -2.
[ O.N. of Pt ] + [
O.N. of Cl ] X 6 = -2
= [O.N. of Pt ]
+ [ 6 X (-1) ]
= -2
= O.N. of Pt
= - 2 + 6 = + 4
: . Oxidation number of Pt in PtCl62- is + 4.
KEY POINTS
Oxidation
·
Loss of electron.
·
Increase in oxidation number of oxidized species.
Reduction
·
Gain of electron.
·
Decrease in oxidation number of reduced species.
Redox reaction
·
Oxidation and reduction occur simultaneously together.
·
Simultaneous loss and gain of electron.
·
Simultaneous increase and decrease in the oxidation number.
Oxidizing agent (oxidant)
·
Causes oxidation of other.
·
Accept electron.
·
It self undergoes reduction.
Reducing agent (Reductant)
·
Causes reduction of other.
·
Donates electron.
·
It self undergoes oxidation.
BALANCING OF REDOX REACTIONS
Two
important methods are used for systematic balancing of redox reactions.
A] Oxidation number method
Step I
Write
the unbalanced net equation for the redox reaction. Assign the oxidation number
to all the atoms in both, the reactant and the product.
Step II
Identify
the atoms that have changed oxidation number. Draw bracket to connect atoms of
the elements that are oxidized and reduced.
Step III
Calculate
the increase or decrease in the oxidation number per atom of molecule. If
increase and decrease in oxidation number is not equal, then equalize them by
multiplying with a suitable number.
Step IV
Balance
hydrogen and oxygen. For balancing oxygen atoms add water molecules to the side
containing less oxygen atoms. Balancing of hydrogen atoms depends upon the
medium acidic or basic.
i)
For acidic solutions, add H+ ions to the side deficient in hydrogen
atoms.
ii)
For basic solutions, add H2O molecule to the side deficient in
hydrogen atoms and simultaneously add equal number of OH- ions on
the other side of the equation.
Step V
Check
the balance equation to make sure that the reaction is balanced with respect to
both the number of atoms of each element and charge.
Illustrative example
Balance the following redox equation by oxidation number method.
H2SO4(aq) + C(s) →
CO2(g) + SO2(g) + H2O(l)
Solution :
Step I
The
oxidation number of all the atoms are as follows :
H2SO4 +
C → CO2 +
SO2 + H2O
↑ ↑
+1
+6 -2 0 +4 -2 +4 -2 +1 -2
Step II
Less of 4e-(oxidation)
H2SO4 +
C → CO2 +
SO2 + H2O
+6 0 +4
+4
Gain of 2e- (Reduction)
Step III
Increase
in oxidation number :
C
(O) →
C (+4), net increase = +4
Decrease
in oxidation number :
S
(+6) →
S (+4), net decrease = -2
To
make total increase and decrease equal the net decrease must be multiplied by
2. Hence the coefficient 2 is required on both the side for S.
2 H2SO4 +
C → CO2 +
2SO2 + H2O
Step IV
Balance
the equation for O atoms by adding H2O molecule to the side with
less O atoms.
2
H2SO4 + C
→ CO2 +
2SO2 + H2O
H
atoms have already been balanced.
Step V
In
reaction the number of atoms and charge is balanced, thus the balanced reaction
is
2
H2SO4(aq) + C(g) →
CO2(g) + 2SO2(g) + H2O(l)
B] lon-electron method (Half reaction method)
Step I
Write
the unbalanced equation for the redox reaction and assign the oxidation numbers
to all the atoms in the reactants and the products.
Step II
Divide
the equation into two half equation. One half is oxidation in which oxidation
number of oxidized species increases and other half is reduction in which
oxidation number of reduced species decreases.
Step III
Balance
the atoms except O and H in each half equation balance O atom by adding H2O
to the side with less O atoms.
Step IV
Balance
H atoms by adding H+ ions to
the side with less H atoms.
Step V
Balance
the charges by adding appropriate number of electrons to the right side of
oxidation half equation and to the left of the reduction half equation.
Step VI
Multiplying
half equations by suitable factors to equalize the number of electrons in the
two half equations. Then add two half equations and cancel the number of
electrons on both the sides of the equation.
Step VII
If
the reaction occurs in basic medium then add OH- ions, equal to the
number of H+ ions, on both the side of the equation, H+ and
OH- ion on same side combine to give H2O molecule.
Step VIII
Check
the equation is balanced for both, the atoms and charges.
Illustrative example
Balance
the following redox equation by ion electron method. The reaction occurs in
acidic medium.
Mn2+(aq) + ClO-3(aq) →
MnO2(s) + ClO2(aq)
Solution :
Step I
Write
the unbalanced equation and assign oxidation numbers to all atoms.
Gain of 2e- (Reduction)
Mn2+(aq) + ClO-3(aq) →
MnO2(s) + ClO2(aq)
+2 +5 -2 +4 -2
+4 -2
Less
of 4e-(oxidation)
Step II
Divide
the equation into two half equation such that one half is oxidation and other
half is reduction.
Oxidation
half equation :
Mn2+ →
MnO2
Reduction
half equation :
ClO-3 →
ClO2
Step III
Balance
the each half equations for O atoms by adding H2O to the side with
less O atoms.
Oxidation
: Mn2+ +
2H2O → MnO2
Reduction
: ClO-3 →
ClO2 + H2O
Step IV
Balance
H atoms by adding H+ ions to the side with less H atoms
Oxidation
: Mn2+ +
2H2O → MnO2 + 4H+
Reduction
: ClO-3 + 2H+ →
ClO2 + H2O
Step V
Balance
the charge on both side of equation by adding appropriate number of electron.
Oxidation
: Mn2+ +
2H2O → MnO2 + 4H+ +
2e-
Reduction
: ClO-3 +
2H+ + e- →
ClO2 + H2O
Step VI
Multiply
reduction half equation by 2 to equalize the number of electrons in two half
equations. Then add two half equations.
Mn2+ +
2ClO-3
→ MnO2 +
2ClO2
Step VII
The
reaction has equal number of atoms and charge, thus the reaction is balanced.
Mn2+ +
2ClO-3
→ MnO2 +
2ClO2
PROBLEMS FOR PRACTICE
Balance the following redox equations by oxidation number method.
a)
Ag +
NO-3 → Ag+ + NO2 (Acidic medium)
b)
Fe2+ + BrO-3 → Fe3+ + Br- (Acidic Medium)
c)
MnCl2 + HO-2 →
Mn(OH)3 + Cl- (Basic Medium)
d)
Mn2+ + H2O2 → MnO2 + H2O (Basic Medium)
Balance the following redox equations by ion - electron method.
a)
SO2 + Fe3+ → Fe2+ + SO42- (Acidic medium)
b)
2HgO →
2Hg + O2 (Acidic Medium)
c)
S2O32-
+ I2 → S4O62- + I- (Basic Medium)
d)
SeO32- + Cl2 → SeO42- + Cl- (Basic Medium)
APPLICATIONS OF REDOX REACTIONS
1) Combustion
Burning
of a substance by oxidation with oxygen in air is called combustion. Combustion
involves redox reactions.
2) Bleaching
Decolourization
or lightening of coloured materials uses redox reaction and is called bleaching.
3) Batteries
The
electricity produced in batteries or galvanic cells is due to redox reactions occurring
in them.
4) Metallurgy
The
extraction and purification of metals uses redox reactions in different steps.
5) Corrosion
The
corrosion is the destruction of metals by oxidation.
For example,
Rusting
of iron is its oxidation by oxygen of air in presence of moisture.
4Fe +
3O2 H2O →
2Fe2O3 . H2O
Corroded
iron
6) Respiration
The
process of breathing and using oxygen is biological redox reactions and is
called respiration. you read these notes on CHEMISTRYNOTESINFO & this is 11th class chemistry notes.

%20(1).png)
