S Block Elements Class 11
OCCURRENCE OF S BLOCK ELEMENTS
S – Block elements do not occur in nature in free state. Among the alkali
metals sodium and potassium are abundant and lithium, rubidium and cesium have
lower abundance. Among the alkaline earth metals magnesium and calcium are
abundant in the earth crust. Radium is rarest of all.
GROUP – 1 ELEMENTS : ALKALI METALS
The general electronic configuration of group – 1 element is ns1.
All alkali metals have one valance electron hence form monovalent M+
ions, and are highly reactive. Group – 1 consists of following elements.
Hydrogen (H), Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium
(S) and Francium (Fr).
The electronic configuration of group – 1 elements is as
follows –
|
Elements |
Symbol |
Atomic Number |
Electronic configuration in condensed form |
|
Hydrogen |
H |
1 |
1s1 |
|
Lithium |
Li |
3 |
[He] 2s1 |
|
Sodium |
Na |
11 |
[Ne] 3s1 |
|
Potassium |
K |
19 |
[Ar] 4s1 |
|
Rubidium |
Rb |
37 |
[Kr] 5s1 |
|
Cesium |
Cs |
55 |
[Xe] 6s1 |
|
Francium |
Fr |
87 |
[Rn] 7s1 |
ANOMALOUS PROPERTIES OF LITHIUM
Amongst alkali metals, lithium has smallest size and highest polarizing
power (charge / radius ratio) due to this reason lithium behaves different from
other elements of group-1 (alkali metals). It shows following anomalous
properties.
·
Lithium is hard metal, its melting point and boiling point
are highest among the group.
·
Lithium is least reactive among all alkali metals and it is
the strongest reducing agent.
·
Lithium hydride is the stable amongst all the alkali metal
hydrides.
·
LiOH is weak base while hydroxides of other alkali metals
behave as strong bases.
·
Lithium nitrate when heated gives lithium monoxide(Li2O).
While other alkali metal nitrates decompose to give corresponding nitrites.
·
Lithium combines with ammonia to form lithium amide (Li2NH)
while other alkali metals form amides of the general formula MNH2
(where M = Na, K, Rb, Cs).
DIAGONAL RELATIONSHIP OF LITHIUM WITH MAGNESIUM
The element lithium of second row shows similar properties with its
diagonally opposite member magnesium of third row. This is known as diagonal
relationship. The diagonal relationship is due to the similarity in ionic
sizes and polarizing power (i.e. charge/radius ratio) of lithium and magnesium.
Also electronegativity of Li (1.00) and Mg (1.20) are not much different.
PERIODIC TRENDS OF ALKALI METALS
1. Atomic and ionic radius of S Block Elements
Alkali metals have the
largest atomic and ionic radius in their respective periods. On moving down the
group, the size goes on increasing due to the presence of extra shells hence
the atomic and ionic radius also increases.
2. Ionization enthalpy or
energy
Ionization enthalpies of
alkali metals are low and decrease down the group from Li to Cs. This is due to
increase in atomic number, size, nuclear charge and increase in screening
effect.
3. Electronegativity
The electronegativity of
the alkali metals is very low due to their electropositive character. The
electronegativity decreases from Li to Cs as the electropositive character
increases.
4. Melting and boiling point
Alkali metals have very
low melting and boiling points due to presence of weak intermetallic bonds. It
decreases on moving down the group.
5. Oxidation States
Due to the presence of
only one electron in the valence shell, they exhibit only +1 oxidation state or oxidation number.
S BLOCK ELEMENTS CHEMICAL REACTIVITY
The alkali metals are highly reactive
due to their large size and low ionization enthalpy.
1. Reactions of S-block elements with oxygen
(air)
Alkali metals burns
vigorously in oxygen to form oxides. Lithium forms monoxide (Li2O)
and sodium forms peroxide, (Na2O2).
4Li + O2 → 2Li2O (Oxide)
2Na + O2 → Na2O2 (Peroxide)
The other elements form
super-oxides.
M + O2
→ MO2 (Superoxide)
(where, M = K, Rb, Cs)
2. Reaction of S-block elements with water
The alkali metals react
with water to form corresponding hydroxides and evolve hydrogen gas
(Dihydrogen). (M = Li, Na, K, Rb, Cs)
2M + 2H2O →
2MOH + H2 ↑
3. Reaction of S-block elements with hydrogen (Dihydrogen)
The alkali metals react
with dry hydrogen at high temperature (673K) to form corresponding hydrides.
2M + H2 →
2MH
4. S-block elements Reaction with halogens
All the alkali metals
react vigorously with halogen to form their respective ionic crystalline
halides with general formula M+X- where M = Na, K, Rb, Cs
and X = Cl, Br, I and F.
2M + X2 Δ → 2M+X-
SOME IMPORTANT COMPOUNDS OF SODIUM
1] Sodium carbonate (washing soda, Na2CO3)
Sodium carbonate is usually prepared by a
process known as the ammonia–soda process or Solvay process. Raw materials are
NaCl, NH3 and limestone (for CO2).
The process involves the following
steps
Step – I
CO2 gas bubbled through a
solution of NH3 to form NH4HCO3 i.e. ammonium
hydrogen carbonate.
NH3 + H2O + CO2 → NH4HCO3
Step – II
Sodium hydrogen carbonate (NaHCO3)
precipitates out because of the common ion effect caused due to the presence of
excess NaCl.
NH4HCO3 +
NaCl → NaHCO3 +
NH4Cl
Step III
The precipitate NaHCO3 is
then filtered of and ignited to get sodium carbonate (Na2CO3)
2NaHCO3 Δ → Na2CO3 + CO2 + H2O
Properties of Na2CO3
Sodium carbonate crystallizes from
water as a decahydrate (sodium carbonate decahydrate, Na2CO3.10H2O,
also known as washing soda).Above 373K, the monohydrate from becomes completely
anhydrous and change to a white powder called soda ash.
Na2CO3.H2O above 373K → Na2CO3 + H2O
(Anhydrous)
Uses of Na2CO3
i.
It is used in the manufacture of glass soap, borax and
caustic soda.
ii.
It is used in water softening in laundry and cleaning.
iii.
It is used in paper, paints and textile industries.
2] Sodium hydroxide (caustic soda, NaOH)
Commercially sodium hydroxide is
prepared by the electrolysis of sodium chloride in Costner – Kellner cell. It
is also prepared by adding calcium hydroxide to the solution of sodium
carbonate.
Na2CO3 +
Ca(OH)2 → 2NaOH
+ CaCO3 ↑
Properties of NaOH
Sodium hydroxide is a deliquescent,
white crystalline solid. It is readily soluble in water and forms a strong
alkaline solution.
The solution of sodium hydroxide at
the surface reacts with CO2 in the atmosphere to form Na2CO3.
2NaOH +
CO2 ↔ Na2CO3 + H2O
Uses of NaOH
i.
It is useful in purification of bauxite and to manufacture ofsoap, paper, artificial silk and a number of chemicals.
ii.
It is useful in petroleum refining and textile industries.
iii.
It is important as laboratory reagent and in preparation of
pure fats and oils.
3] Sodium Chloride (NaCl)
It is also known as common salt or
table salt. It is mainly obtained by the evaporation of sea water. Crude sodium
chloride thus obtained contains CaSO4, CaCl2, MgCl2
as impurities and is deliquescent. Pure NaCl is obtained by passing HCl gas
through saturated solution of crude NaCl. Due to common ion effect NaCl gets
precipitated.
Properties of Sodium Chloride (NaCl)
It is white crystalline solid. It has
melting point 1081K and boiling point 1713K. It has solubility of 36gram per
100gram of water at 273K. NaCl when heated with concentrated H2SO4
and MnO2 it liberates chlorine gas.
2NaCl
+ MnO2 + 2H2SO4 → MnSO4 + Na2SO4 + 2H2O + Cl2 ↑
Uses of Sodium Chloride (NaCl)
i.
It is used as a common salt or table salt for domestic
purpose and preservative for meat, fish etc.
ii.
It is used for the preparation of Na2O2,
NaOH, Na2CO3 etc.
4] Sodium hydrogen carbonate (NaHCO3)
It is also known as sodium
bicarbonate and baking soda. It is made by saturating a solution of sodium
carbonate with CO2.
Na2CO3 + H2O +
CO2 → 2NaHCO3
Properties Sodium hydrogen carbonate (NaHCO3)
It is white crystalline solid,
sparingly soluble in water. On heating, it gives Na2CO3
and CO2.
2NaHCO3 373K → Na2CO3 + CO2 + H2O
It’s aqueous solution is alkaline due
to hydrolysis.
NaHCO3 + H2O →
NaOH + H2CO3
Uses Sodium hydrogen carbonate (NaHCO3)
i.
It is used in fire extinguisher and for baking of cakes
breads etc.
ii.
It is also used as medicine to minimize the acidity of
stomach i.e. antacid.
BIOLOGICAL IMPORTANCE OF SODIUM AND POTASSIUM
Sodium and potassium are very important and play a vital role in biological system. Our bodies obtain them from fruits and vegetable. Common salt is the most important source of sodium in the diet. Their ions maintain the sensitivity of nerves and control muscles.
Deficiency of sodium shows reduction in fat deposit, atrophy, lung infection retarted
bone growth, reduces blood pressure etc. Deficiency of potassium reduces heart
beats. Hypertrophy of kidneys and paralysis of muscle.
GROUP – 2 ELEMENTS : ALKALINE EARTH METALS
The general electronic configuration
of group–2 elements is ns2. All alkaline earth metals have
two valance electrons, hence form divalent M2+ ions, and are highly
reactive like alkaline metals. Group – 2 consists of following elements.
Beryllium (Be), Magnesium (Mg),
Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
The electronic configuration of group – 2 elements is an follows –
|
Elements |
Symbol |
Atomic Number |
Electronic Configuration |
|
Beryllium |
Be |
4 |
[He] 2s2 |
|
Magnesium |
Mg |
12 |
[Ne] 3s2 |
|
Calcium |
Ca |
20 |
[Ar] 4s2 |
|
Strontium |
Sr |
38 |
[Kr] 5s2 |
|
Barium |
Ba |
56 |
[Xe] 6s2 |
|
Radium |
Ra |
88 |
[Rn] 7s2 |
ANOMALOUS PROPERTIES OF BERYLLIUM
Beryllium, the first member of the
group – 2 metals, shows anomalous properties as compared to magnesium and rest
of the members of group-2. It is due to its small size and highest polarizing
power. It shows following anomalous properties.
·
Beryllium is hard metal while other alkaline earth metals are
soft.
·
The melting, boiling points and ionization enthalpy of Be are
highest of all the alkaline earth metals.
·
Be forms covalent compounds while other members form ionic
compound.
·
Be does not liberate H2 from acids while other
metals liberate H2.
DIAGONAL RELATIONSHIP BETWEEN BERYLLIUM AND ALUMINIUM
The element beryllium is the first
member of group – 2 which shows similar properties with its diagonals opposite
element aluminum of group – 13. This diagonal relationship is due to similarity
in ionic sizes and polarizing power.
PERIODIC TRENDS OF ALKALINE EARTH METALS
1. Atomic and ionic radius
The atomic and ionic radii
of the alkaline earth elements are smaller than those of the corresponding
alkaline metals of the same periods. The atomic and ionic radius increases down
the group with increase in atomic number.
2. Ionization enthalpy or
energy
Ionization enthalpies of
alkaline earth metals are higher than those of alkali metals. Ionization
enthalpy decreases down the group.
3. Electronegativity
The electronegativity of
group–2 elements decreases down the group. Their electronegativity values are
higher than those of alkali metals.
4. Melting and boiling point
The melting and boiling
points are not regular in the group, mainly due to different crystal structure
of the metals. However, these are higher than those of alkali metals.
5. Oxidation states
Due to the presence of
only two electrons in the valence shell, they exhibit only +2 oxidation state.
CHEMICAL REACTIVITY
Due to low ionization enthalpy,
alkaline earth metals are fairly reactive. However, their chemical reactivity
is lower than those of alkali metals.
1. Reaction with oxygen
With pure oxygen, Be, Mg
and Ca form oxides whereas Ba, Sr and Ra form peroxides.
2M + O2 Δ → 2MO
(M = Be, Mg or Ca)
Metal oxide
M + O2 Δ → MO2 (M = Ba, Sr or Ra)
Metal peroxide
The affinity of metals
towards oxygen increases down the group.
2. Reaction with water
Alkaline earth metals
react with water to form metal hydroxides and evolve hydrogen gas. The chemical
reactivity with water increases from Mg to Ba.
M + 2H2O →
M(OH)2 + H2 ↑
(M = Mg, Ca, Sr or Ba)
3. Reaction with hydrogen
Except Be all the alkaline
earth metals react with hydrogen on heating to form metal hydride of the general
formula, MH2.
M + H2 Δ → MH2 (M = Mg, Ca, Sr or Ba)
4. Reaction with halogens
All the alkaline earth
metals react with halogens at high temperature to form their halides.
M + X2 →
MX2 (X = F, Cl, Br,
I)
IMPORTANT COMPOUNDS OF CALCIUM
1] Calcium oxide or quick lime (CaO)
It is prepared commercially by
heating limestone (CaCO3) in a reverberatory kiln at 1070 to 1270K.
Properties Calcium oxide or quick lime (CaO)
Calcium oxide is a white amorphous
solid. It has a melting point of 2870K. When it is exposed to atmosphere, it
absorbs moisture and carbon dioxide.
CaO
+ H2O →
Ca(OH)2
CaO
+ CO2 →
CaCO3
Uses Calcium oxide or quick lime (CaO)
i.
It is used in the manufacture of sodium carbonate from
caustic soda.
ii.
It is an important primary material for large scale
production of cement.
2] Calcium carbonate (CaCO3)
Calcium carbonate occurs in nature in
several forms like chalk, lime stone, marble state, calcite etc. It is prepared
by passing carbon dioxide through slaked lime.
Ca(OH)2 +
CO2 → CaCO3 + H2O
It can also be prepared by adding
sodium carbonate to calcium chloride.
CaCl2 +
Na2CO3
→ CaCO3 +
2NaCl
Properties
It is white fluffy powder. It is
almost insoluble in water. When CaCO3 is heated to 1200K, it
decomposes to evolve CO2.
It reacts with dilute acid
to liberate carbon dioxide.
CaCO3 +
H2SO4
→ CaSO4 +
H2O + CO2 ↑
Uses
i.
It is used in the manufacture of quick lime and in building
material in the form of marble.
ii.
It is used as antacid, mild abrasive of tooth paste,
constituent of chewing gum.
3] Calcium hydroxide or slaked lime (Ca(OH)2)
It is prepared by adding
water to quick lime.
CaO + 2H2O →
Ca(OH)2 + H2 ↑
It is also prepared by
treating calcium chloride with caustic soda.
CaCl2 +
2NaOH → Ca(OH)2 +
2NaCl
Properties
It is white amorphous powder,
sparingly soluble in water and the aqueous solution is called lime water. When
CO2 is passed through lime water, it turns milky
Ca(OH)2 + CO2 →
CaCO3 + H2O
Milky
The milkiness disappears
on passing CO2 gas in excess.
CaCO3 + CO2 + H2O →
Ca(HCO3)2
Soluble
milkiness disappears
Uses
i.
It is used to prepare mortar which is an important building
material.
ii.
It is used glass and tanning industry also to prepare
bleaching powder and for purification of sugar.

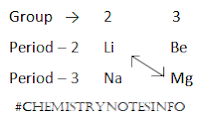

%20(1).png)

Thanks
ReplyDeletewelcome
DeleteThank you so much for sharing all this wonderful info with the how-to's!!!! It is so appreciated!!! You always have good humor in your posts/blogs. So much fun and easy to read!
ReplyDeleteSoda PDF Crack
ON1 Photo RAW Crack
Advance Bulk Mailer Pro Crack
Tomabo MP4 Downloader Pro Crack
WinRAR Crack