Alcohols Phenols and Ethers Class 12 Chemistry Notes
Alcohols Phenols and Ethers Class 12 Chemistry Noteswww.ChemistryNotesInfo.com
ALCOHOLS
Alcohols are the hydroxyl derivatives of hydrocarbons in which one or more hydrogen atoms are replaced by –OH group.
e.g. 1) CH3OH (Methyl Alcohol)
2) CH3 – CH2OH (Ethyl Alcohol)
Classification of Alcohols
On the basis of the number of hydroxyl groups (-OH) present in the molecule, alcohols are classified as:
1) Monohydric alcohols:
They have only one hydroxyl (-OH) group.
e.g. CH3OH)
2) Dihydric alcohols/glycols/diols:
They have two hydroxyl groups. eg.
3) Trihydric Alcohols/glycerol’s/triols :
They have three hydroxyl groups.
4) Polyhydric alcohols :
They have more than three hydroxyl groups.
Monohybrid alcohols are further classified according to the type of hybridization of the carbon atom to which the hydroxyl group is attached.
A) Alcohols containing C sp3 – OH bond –
In these alcohols, -OH group is attached to SP3 hybridised carbon atom of an alkyl, allyl or benzylic group. They are further classified as primary, secondary and tertiary alcohols according to number of carbon atom.
e.g. 1) CH3 – CH2 – OH (Ethyl Alcohol)
Allylic Alcohols
In these alcohols –OH group is attached to a sp3 hybridized carbon atom next to carbon atom.
Benzylic Alcohols
In these alcohols –OH group is attached to a sp2 hybridized carbon atom i.e. vinylic carbon. These alcohols are also called vinylic alcohols.
e.g. CH2 = CH – OH (vinyl alcohol)
B) Alcohols containing Csp2 – OH bond
In these alcohols –OH group is attached to sp2 hybridized carbon atom i.e. vinylic carbon. These alcohols are also called vinylic alcohols.
e.g. CH2 = CH – OH (vinyl alcohol)
Preparation of Alcohols
1) From alkyl halides (Hydrolysis)
Alkyl halides in heating with dilute aqueous alkali give corresponding alcohols.
R – X + NaOH → R – OH + NaX
Alkyl halides Alcohols
e.g. CH3 – Cl + NaOH → CH3 – OH + NaCl
Methyl Chloride Methanol
2) From alkynes (Hydration)
The addition of the water molecule across the double bond in alkenes is called hydration of alkenes. It takes place according to Markownikoff’s rule. Hydration of alkenes is carried out by passing an alkene through cold concentrated Sulphuric acid to give alkyl hydrogen sulphate, which on heating with water gives an alcohol.
3) From carbonyl compound (Reduction)
Hydrogenation of aldehydes and ketones is carried out in the presence of catalysts such as finely divided nickel, platinum or palladium, aldehydes on reduction give corresponding primary alcohol while ketones on reduction give corresponding secondary alcohols.
e.g. i) Ethanol is prepared by hydrogenation at acetaldehyde.
CH3 – CHO + H2 —-Raney Ni 413k→ CH3 – CH2 – OH
CH3 – CHO + H2 —-Raney Ni 413k→ CH3 – CH2 – OH
Acetaldehyde Ethanol
ii) Acetone on hydrogenation gives isopropyl alcohol.
Conversion of carboxylic acids and esters to corresponding primary alcohols reduction by LiAlH4 is preferred.
4) From Grignard reagent
Grignard reagent forms addition compound by nucleophilic attack with aldehydes and ketones, which on hydrolysis with dilute acid yields corresponding alcohol.
e.g. Ethanol is prepared by the action of methyl magnesium bromide on methanol in dry ether followed by the hydrolysis of the addition compound using dilute acid.
Structure of Alcohols
The carbon atom attached to –OH group and oxygen atom of the –OH group are sp3 hybridized. The C – O – H bond angle has an approximate tetrahedral value i.e. around 108.9ᵒ.
Reactions of Alcohols
Alcohol reacts both as nucleophiles and electrophiles. The C–O and O–H bond in alcohols are polar, hence alcohols are quite reactive.
A) Reaction involving breaking of O – H bond
This section of chapter Alcohols Phenols and Ethers contains reaction of alcohols which involve breaking of O-H bond.
i) Action of Metal
Alcohols react with active metals like sodium, potassium, aluminium to give corresponding alcoxide liberating hydrogen gas. These reactions explain acidic nature of alcohols. As number of alkyl group increases, acidic strength of alcohol decreases.
e.g. 2C2H5 – OH + 2Na → 2C2H5 – ONa + H2 ↑
ii) Esterification
When carboxylic acids are heated with alcohols, they give corresponding esters. This process is called Fischer esterification. The reaction is reversible and accumulation of water may reverse the reaction. The forward reaction is called esterification and the backward reaction is called hydrolysis of ester.
B) Reaction involving breaking of C – O bond
This section of chapter Alcohols Phenols and Ethers contains reactions of alcohols which involve breaking of C-O bond.
i) Reaction with hydrogen halide (HX)
- Action of HCl
A catalyst (Lewis base) is required for the reaction of primary and secondary alcohols with HCl. For this purpose, Lucas reagent is used which is composed of conc. HCl and anhydrous zinc Chloride.
e.g. C2H5OH (Ethanol) + HCl anhy. ZnCl2Δ→ C2H5Cl (Chloroethane) + H2O
Action of HBr
Action of HBr
Alcohols when heated with HBr give alkyl Bromides. Since HBr is Stronger than HCl, zncl2 catalyst is not required.
C2H5OH (Ethanol) + HBr — Δ→ C2H5Br (Bromoethane) + H2O
- Action of HI
Alcohols on heating with hydrogen iodide give corresponding alkyl iodides. However, most of the alcohols do not give acceptable yields of alkyl iodides.
ii) Reaction with PX3
Alcohols are converted to alkyl Chlorides by reaction with phosphorus trichloride (PCl3).
e.g. 3C2H5OH (Ethanol) + PCl3 (Phosphorous Trichloride) —Δ→ 3C2H5Cl (Ethyl Chloridde) + H3PO4 (Phosphorus acid)
iii) Dehydration of formation of alkene
Removal of water molecule from an alcohol molecule is called dehydration of alcohol. When alcohol having a β-hydrogen is heated with dehydrating agent like concentrated H2SO4 an alkene is formed by the loss of water.
e.g. A primary alcohol is dehydrated by heating with 95% H2SO4 at 443K.
C2H5OH 95% H2SO4 (443K)→ CH = CH + H2O
Saytzeff’s rule:- In dehydrohalogenation or in dehydration, an alkenes formed by elimination has greater number of alkyl groups attached to doubly bonded carbon atom.
iv) Oxidation of formation of aldehydes and Ketones
Alcohols can be oxidized by various oxidizing agent such as acidified K2Cr2O7 and H2SO4.
a) Oxidation of Primary alcohols
A primary alcohol on oxidation using acidified potassium dichromate (K2Cr2O7) first gives an aldehyde which on further oxidation gives carboxylic acid.
b) Oxidation of secondary alcohols
A secondary alcohol on oxidation using K2Cr2O7 and H2SO4 give a ketone containing same number of carbon atoms as in the alcohol.
PHENOLS
Phenols are organic aromatic, hydroxyl compounds, in which one or more hydroxyl (-OH) groups are directly attached to the aromatic nucleus i.e. benzene like ring. It is represented by Ar-OH. These are Alcohols Phenols and Ethers class 12 chemistry notes by ChemistryNotesInfo.com
Classification of Phenols
Phenols are classified as monohydric, dihydric and trihydric depending upon presence of one, two or three hydroxyl groups (-OH) attached to aromatic ring.
Preparation of Phenols
1) From chlorobenzene (Dow process)
In this method, Chlorobenzene is fused with NaOH at 623K and 32O atmospheric pressure to get sodium phenoxide further it is hydrolyzed using dilute hydrochloric acid to get phenol.
2) From Chlorobenzene (Raschig method)
In this method, phenol is obtained by heating chlorobenzene with steam at 698K using Ca3(PO4)2 or Sio2 as a catalyst.
3) From benzene Sulphonic acid
Benzene is sulphonated with oleum (H2S2O7) and benzene sulphonic acid so formed is concentrated to sodium phenoxide on heating with molten sodium hydroxide. Acidification of sodium phenoxide gives phenol.
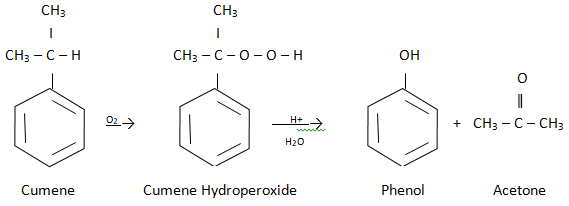
4) From Cumene (commercial method)
Cumene (isopropyl benzene) is oxidized in the presence of air to cumene hydroperoxide. It is converted to phenol and acetone by treating it with dilute acid.
Structure of Phenol
In phenols –OH group is attached to sp3 hybridised carbon atom of an benzene ring. The carbon – oxygen (C – O) bond length is 136 Pm and C – O – H bond angle is 109ᵒ. Phenol has smaller dipole moment.
Reaction of Phenol
Acidity of Phenol
- Reaction with sodium
Phenol reacts with sodium to give sodium phenoxide along with evolution of H2 gas.
- Reaction with NaOH
Phenol dissolves in NaOH to give sodium phenoxide and water.
The Above two reactions show acidic character of phenol. The acidity of phenol is due to its ability to lose hydrogen ion to form phenoxide ions. In a phenol molecule, the hydroxyl group (-OH) is directly attached to SP2 hybridised carbon atom act as an electron withdrawing group.
Electrophilic aromatic substitution
- Halogenation
Bromination of phenol is treated with bromine at low temperature in a solvent, such as carbon disulphide (CS2) or chloroform (CHCl3) a mixture of O – bromophenol and p – bromophenol is obtained, P – bromophenol is major product.
When phenol is treated with bromine water 2,4,6 – tribromophenol is formed with white precipitate.
- Nitration of Phenol
When phenol is treated with dilute nitric acid at room temperature a mixture of O – nitrophenol and P – nitrophenol is obtained and O – nitrophenol is major product.
When phenol is heated with concentrated nitric acid in presence of concentrated sulphuric acid, it gives 2,4,6 trinitrophenol.
- Sulphonation of Phenol
When phenol is treated with concentrated sulphuric acid at room temperature (298K) it gives O – phenol sulphonic acid as major product.
- Kolbe’s reaction
The electrophilic substitution reaction between phenoxide ion and carbon dioxide to give Ortho – Hydroxybenzoic acid is known as Kolbe’s reaction.
- Reimer – Tiemann reaction
On treating phenol with Chloroform in the presence of sodium hydroxide, α – CHO group is introduced at ortho position of benzene ring. This reaction is known as Reimer – Tiemann reaction.
- Reaction with zinc dust
When phenol is heated with zinc dust, it gives benzene and zinc oxide.
- Oxidation
Phenol is oxidized by chromic acid to a diketone, benzoquinone.
ETHERS
Ethers are derivatives of hydrocarbons in which a hydrogen atom is replaced by an alkoxy (-OR) or an aryloxy (- OAr) group. They are represented as R – O – R.
e.g. CH3 – O – CH3
Dimethyl Ether
Classification of ether
1) Simple or symmetrical ethers
The ether’s in which both the alkyl or aryl groups attached to oxygen atom are same, are called simple ethers.
e.g. CH3 – O – CH3
Dimethyl Ether
2) Mixed or unsymmetrical ethers
The ethers, in which the two alkyl or aryl group attached to oxygen atom are different, are called mixed ethers.
e.g. CH3 – O – C2H5
ethyl methyl ether
Structure of ether
In ether oxygen atom is SP3 hybridized. C – O – C bond angle (111.7ᵒ) is slightly greater than tetrahedral angle. C – O bond length is 141 Pm.
Metamerism
Ethers having same molecular formula but different alkyl groups attached on either side of the oxygen atom are called metamers of each other. This phenomenon is called metamerism.
e.g. Molecular Formula – C4H10O
CH3 – CH2 – O – CH2 – CH3 Diethyl ether, and
CH3 – O – CH2 – CH2 – CH3 Methyl n – propyl ether.
Methods of preparation of ethers
1) By dehydration of alcohols
When excess of ethyl alcohol is distilled with concentrated sulphuric acid at 443K it gives dimethyl ether. The formation of ether by dehydration of alcohol is a nucleophilic bimolecular reaction (SN2)
2) From alkyl halides (Williamson synthesis)
Simple as well as mixed ethers can be prepared, when an alkyl halide is heated with alcoholic sodium or potassium alkoxide.
Chemical properties of ethers
1) Cleavage of C – O bond by action of HX
a) Cold simple ether reacts with HX to give one molecule of alkyl halide and one molecule of an alcohol, while when heated gives two molecule of alkyl halide.
b) By reacting with HX cold mixed ether gives generally a lower alkyl iodide and a higher alcohol while when heated it gives two different alkyl halides.
2) Hydrolysis
Simple ethers on heating with dilute sulphuric acid under pressure give alcohol.
3) Electrophilic substitution
In this section of chapter Alcohols Phenols and Ethers, we study about electrophilic substitution reactions of ether.
a) Halogenation
Anisole undergoes bromination with bromine in acetic acid.
b) Friedal – crafts reaction
Alkyl and acyl groups are introduced at ortho and para position in anisole on reaction with alkyl halide and acyl chloride respectively in presence of anhydrous aluminium chloride.
c) Nitration
Anisole reacts with nitrating mixture to give a mixture of ortho and para nitro derivatives.
Uses of diethyl ether
It is used for industrial solvent for oils, fats, gum, and resin and in Grignard reagents. It is also used as refrigerant.
Like Subscribe and Share these Alcohols Phenols and Ethers chemistry notes by Chemistry Notes Info with your friends…






































%20(1).png)
